Label: FABRAZYME- agalsidase beta injection, powder, lyophilized, for solution
- NDC Code(s): 58468-0040-1, 58468-0041-1
- Packager: Genzyme Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated July 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FABRAZYME® safely and effectively. See full prescribing information for FABRAZYME®. FABRAZYME® (agalsidase beta) for injection ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HYPERSENSITIVITY REACTIONS INCLUDING ANAPHYLAXIS
Patients treated with enzyme replacement therapies have experienced life-threatening hypersensitivity reactions, including anaphylaxis. Anaphylaxis has occurred during the early course of enzyme replacement therapy and after extended duration of therapy. Initiate FABRAZYME in a healthcare setting with appropriate medical monitoring and support measures, including access to cardiopulmonary resuscitation equipment. If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, discontinue FABRAZYME and immediately initiate appropriate medical treatment, including use of epinephrine. Inform patients of the symptoms of life-threatening hypersensitivity reactions, including anaphylaxis and to seek immediate medical care should symptoms occur [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEFABRAZYME® is indicated for the treatment of adult and pediatric patients 2 years of age and older with confirmed Fabry disease.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommendations Prior to FABRAZYME Treatment - Administration of FABRAZYME should be supervised by a healthcare provider knowledgeable in the management of hypersensitivity reactions ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 5 mg or 35 mg of agalsidase beta as a white to off-white, lyophilized cake or powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions Including Anaphylaxis - Life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients treated with enzyme replacement ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: Hypersensitivity Reactions Including Anaphylaxis [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from a pregnancy sub-study within the Fabry Disease registry, post-marketing case reports, and case series with FABRAZYME use during pregnancy ...
-
11 DESCRIPTIONAgalsidase beta is a recombinant human α-galactosidase A enzyme with the same amino acid sequence as the native enzyme. Purified agalsidase beta is a homodimeric glycoprotein with a molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - FABRAZYME (agalsidase beta) provides an exogenous source of α-galactosidase A in Fabry disease patients. Agalsidase beta is internalized and transported into lysosomes ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There are no animal or human studies to assess the carcinogenic or mutagenic potential of FABRAZYME. A study to evaluate the effects of ...
-
14 CLINICAL STUDIESThe safety and efficacy of FABRAZYME were assessed in four clinical studies in patients with Fabry disease and one matched analysis based on data from observational studies. Study 1 was a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFABRAZYME (agalsidase beta) for injection is supplied as a sterile, nonpyrogenic, white to off-white lyophilized cake or powder in single-dose vials. 35 mg vial: NDC 58468-0040-1 - 5 mg vial: NDC ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions Including Anaphylaxis and Infusion-Associated Reactions (IARs) Advise the patient and caregiver that life-threatening hypersensitivity reactions, including ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Genzyme Corporation - 450 Water Street - Cambridge, MA 02141 - A SANOFI COMPANY - U.S. License Number: 1596 - FABRAZYME and Genzyme are registered trademarks of Genzyme Corporation
-
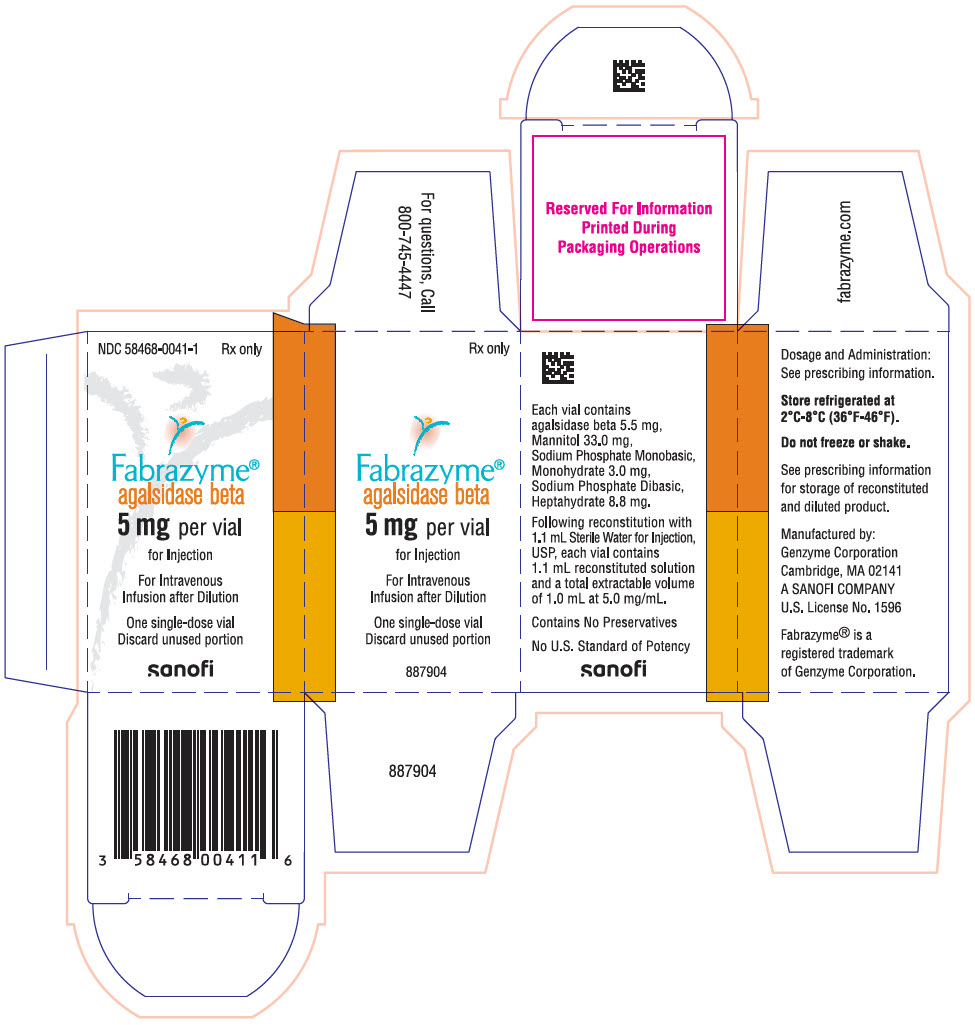
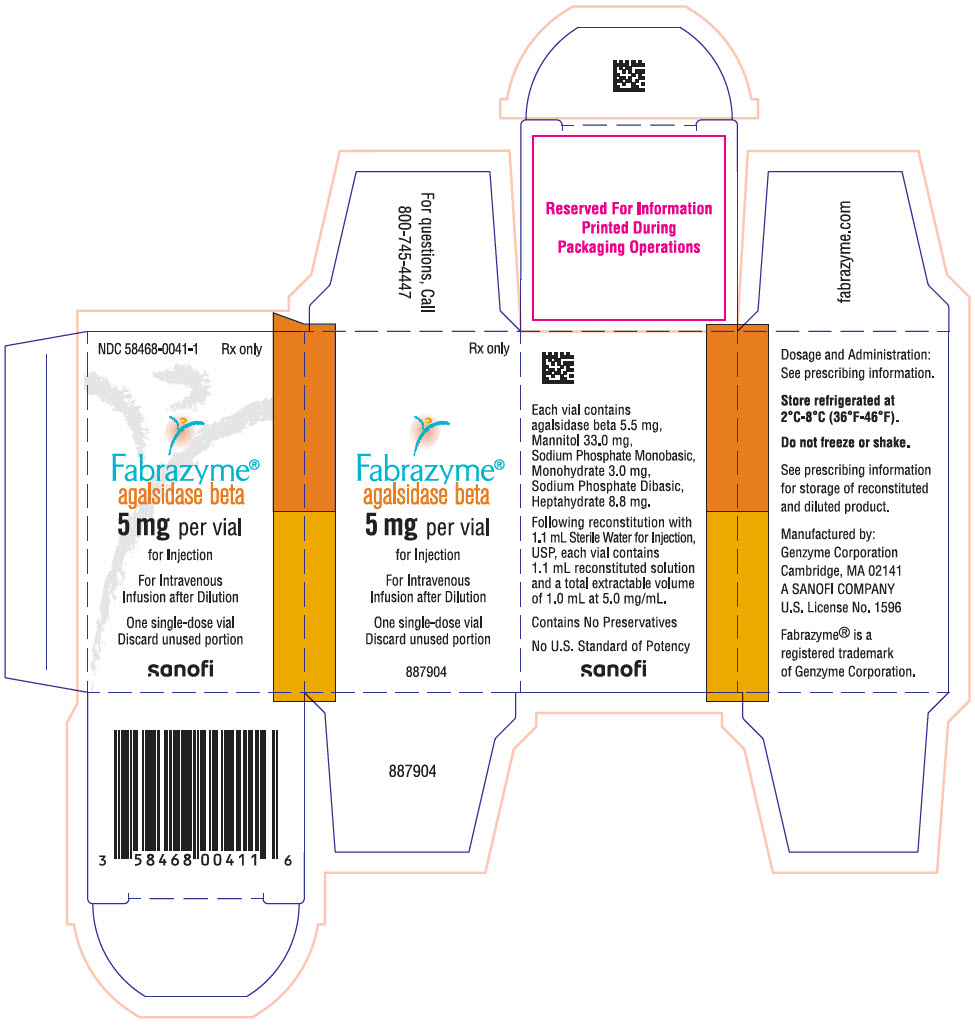
PRINCIPAL DISPLAY PANEL - 5 mg Vial CartonNDC 58468-0041-1 - Rx only - Fabrazyme® agalsidase beta - 5 mg per vial - for Injection - For Intravenous - Infusion after Dilution - One single-dose vial - Discard unused portion - sanofi
-
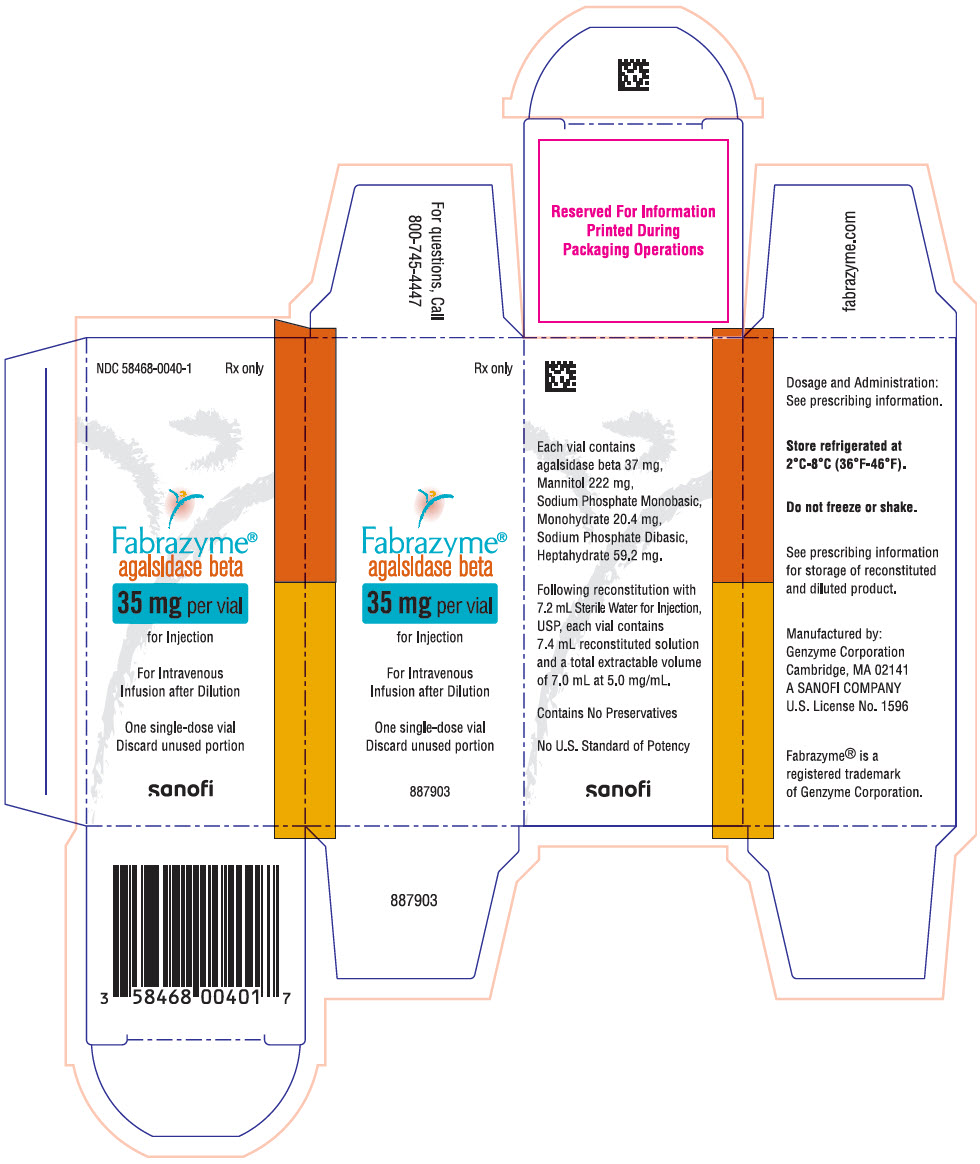
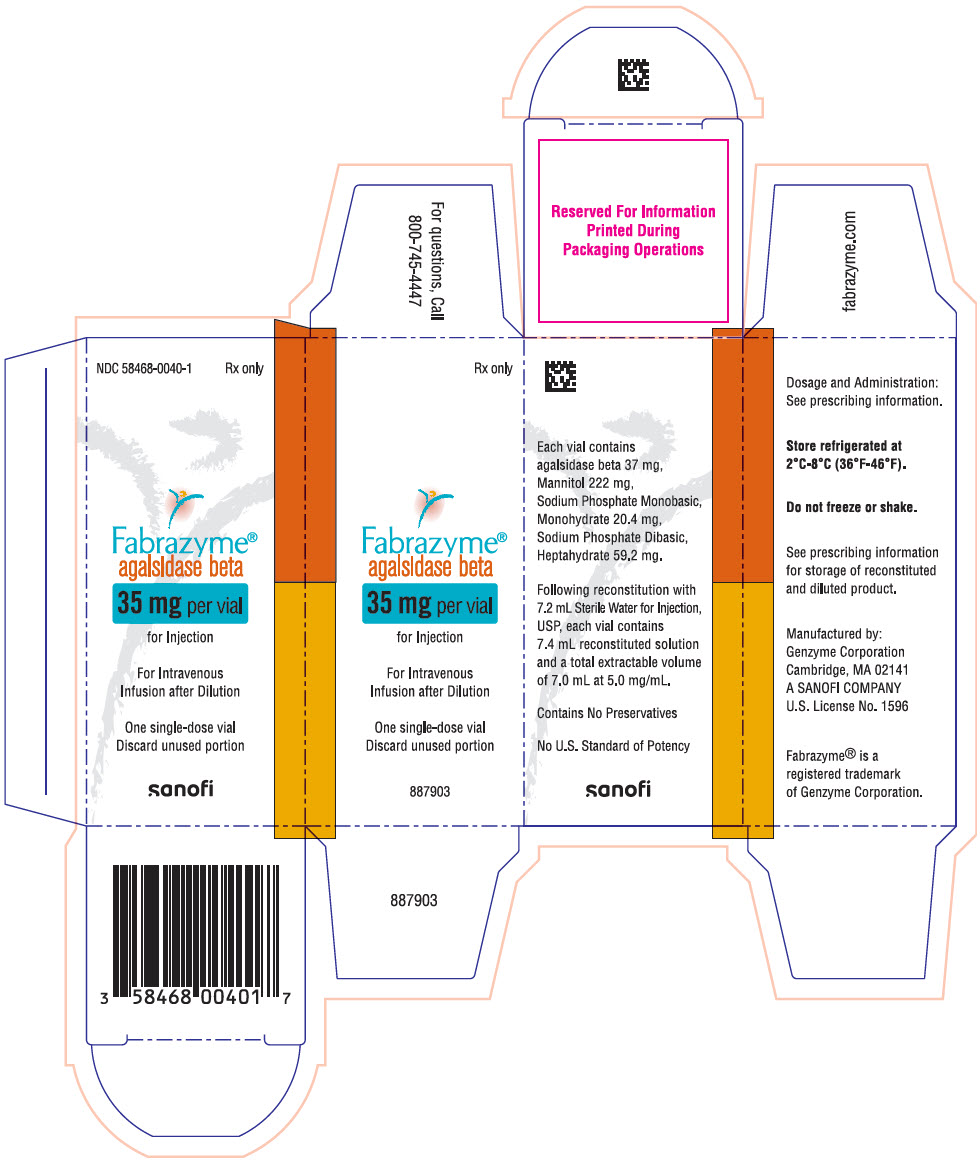
PRINCIPAL DISPLAY PANEL - 35 mg Vial CartonNDC 58468-0040-1 - Rx only - Fabrazyme® agalsidase beta - 35 mg per vial - for Injection - For Intravenous - Infusion after Dilution - One single-dose vial - Discard unused portion - sanofi
-
INGREDIENTS AND APPEARANCEProduct Information