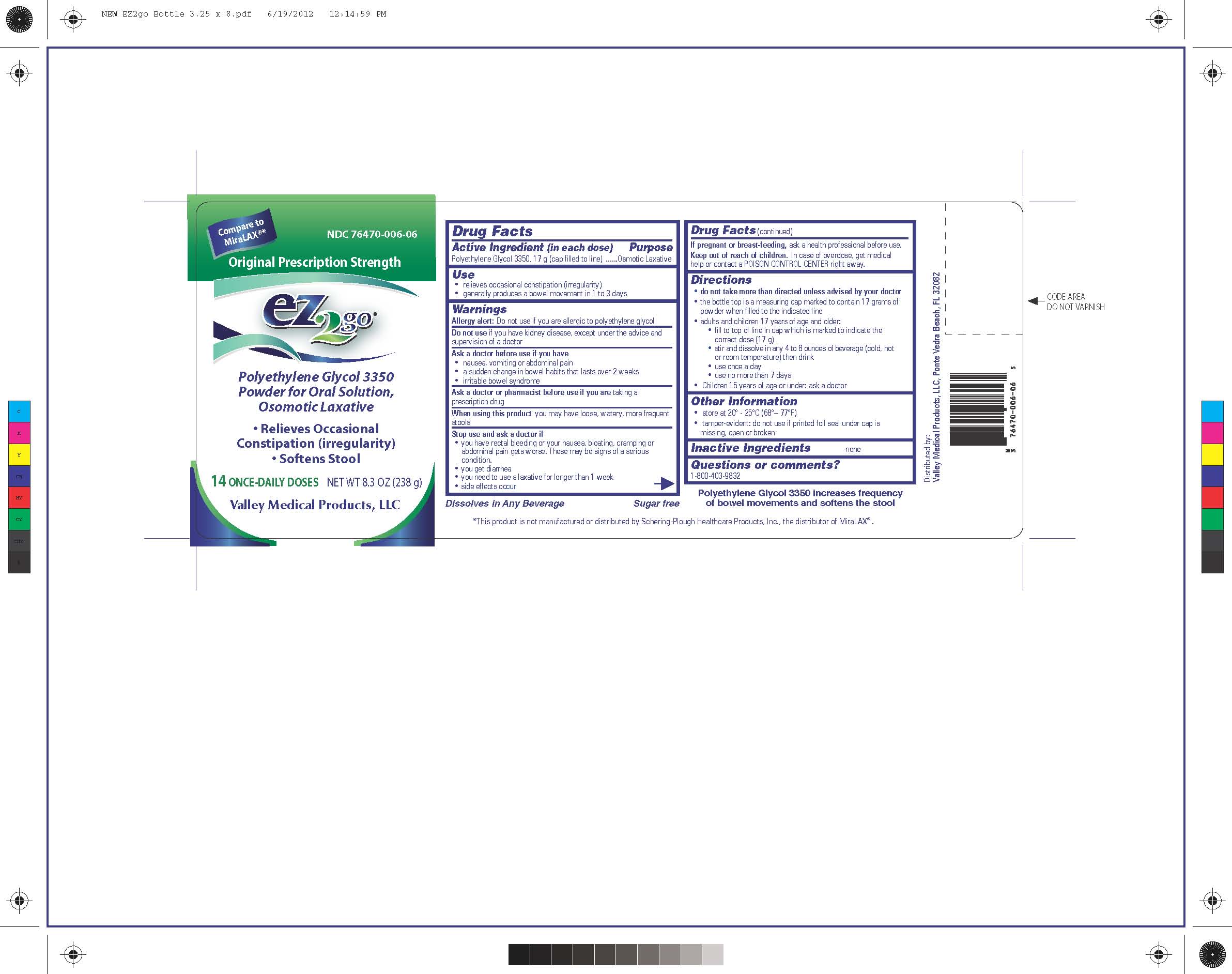
Label: EZ2GO PEG 3350- polyethylene glycol 3350 powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 76470-006-06 - Packager: Valley Medical Products,LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 11, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INACTIVE INGREDIENTInactive Ingredients none
-
ACTIVE INGREDIENTActive Ingredient (in each dose) Polyethylene Glycol 3350, 17 g (cap filled to line)
-
PURPOSEPurpose - Osmotic Laxative
-
WARNINGSWarnings - Allergy Alert: Do not use if you are allergic to polyethylene glycol - Do not use if you have kidney disease, except under the advice and supervision of a doctor ...
-
ASK DOCTORAsk a doctor before use if you have - -nausea, vomiting or abdominal pain - -a sudden change in bowel habits that lasts over 2 weeks - -irritable bowel syndrome - Ask a doctor or pharmacist before use ...
-
STOP USEStop use and ask a doctor if - - you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse.These may be sogns of a serious condition. - you get ...
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children. In case of overdose, get medical help or contact a POISON CONTROL CENTER right away.
-
PREGNANCY OR BREAST FEEDINGIf pregnant or breast-feeding, ask a health professional before use.
-
DOSAGE & ADMINISTRATIONDirections - -do not take more than directed unless advised by your doctor - -the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line - -adults and ...
-
QUESTIONSQuestions or comments? 1-800-403-9832
-
INDICATIONS & USAGEUse - -relieves occasional constipation (irregularity) -generally produces a bowel movement in 1 to 3 days
-
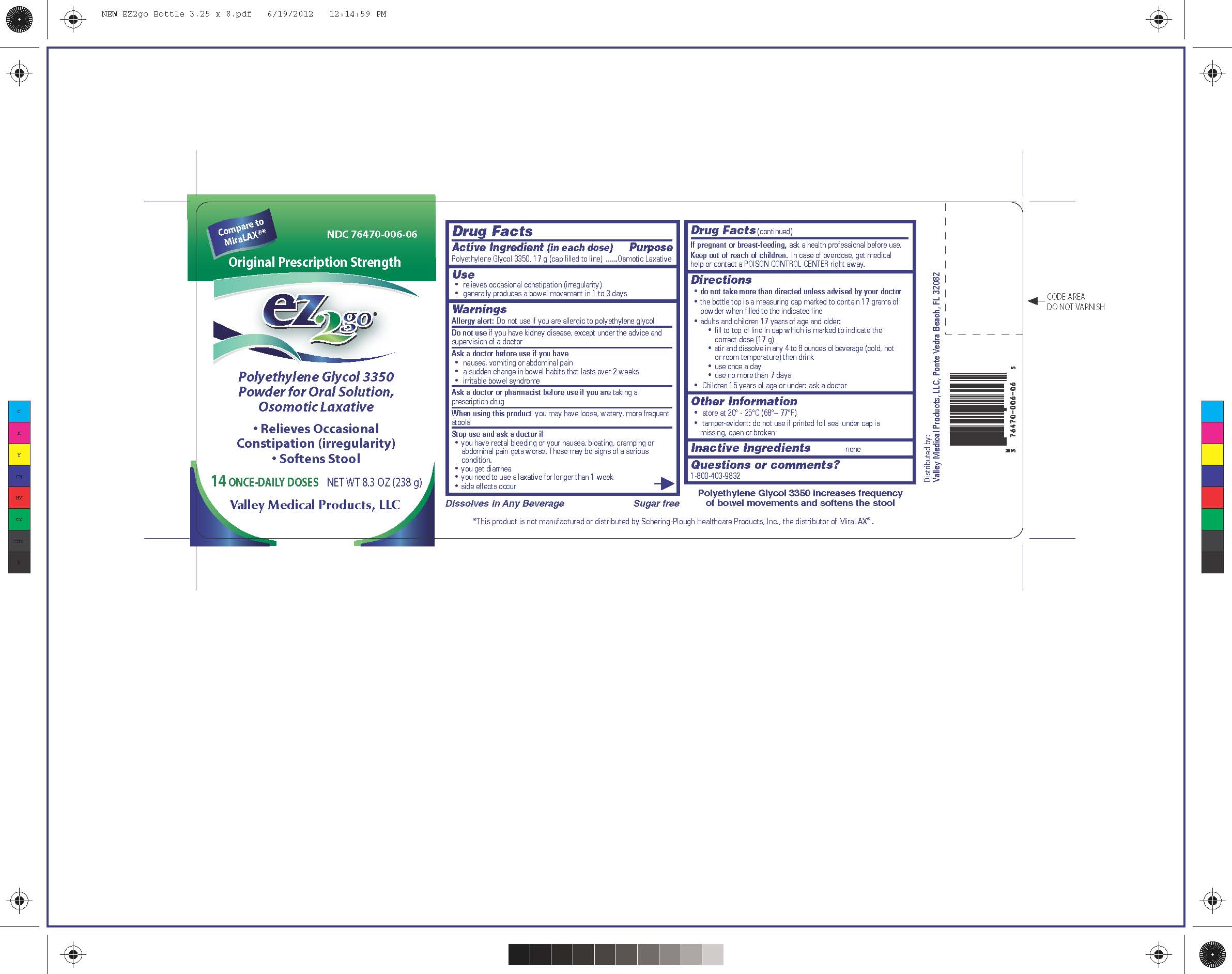
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information