Label: EVOMELA- melphalan injection, powder, lyophilized, for solution
- NDC Code(s): 72893-001-01
- Packager: Acrotech Biopharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EVOMELA® safely and effectively. See full prescribing information for EVOMELA. EVOMELA (melphalan) for injection, for intravenous ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SEVERE BONE MARROW SUPPRESSION, HYPERSENSITIVITY, and LEUKEMOGENICITY
-
Severe bone marrow suppression with resulting infection or bleeding may occur. Controlled trials comparing intravenous (IV) melphalan to oral melphalan have shown more myelosuppression with the IV formulation. Monitor hematologic laboratory parameters. [see Warnings and Precautions (5.1)]
-
Hypersensitivity reactions, including anaphylaxis, have occurred in approximately 2% of patients who received the IV formulation of melphalan. Discontinue treatment with Evomela for serious hypersensitivity reactions. [see Warnings and Precautions (5.4)]
- Melphalan produces chromosomal aberrations in vitro and in vivo. Evomela should be considered potentially leukemogenic in humans. [see Warnings and Precautions (5.5)]
Close -
Severe bone marrow suppression with resulting infection or bleeding may occur. Controlled trials comparing intravenous (IV) melphalan to oral melphalan have shown more myelosuppression with the IV formulation. Monitor hematologic laboratory parameters. [see Warnings and Precautions (5.1)]
-
1 INDICATIONS AND USAGE1.1 Multiple Myeloma-Conditioning Treatment - Evomela is indicated for use as a high-dose conditioning treatment prior to hematopoietic progenitor (stem) cell transplantation in patients with ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage for Conditioning Treatment - The recommended dose of Evomela for conditioning treatment is 100 mg/m2/day administered over 30 minutes by intravenous infusion for 2 ...
-
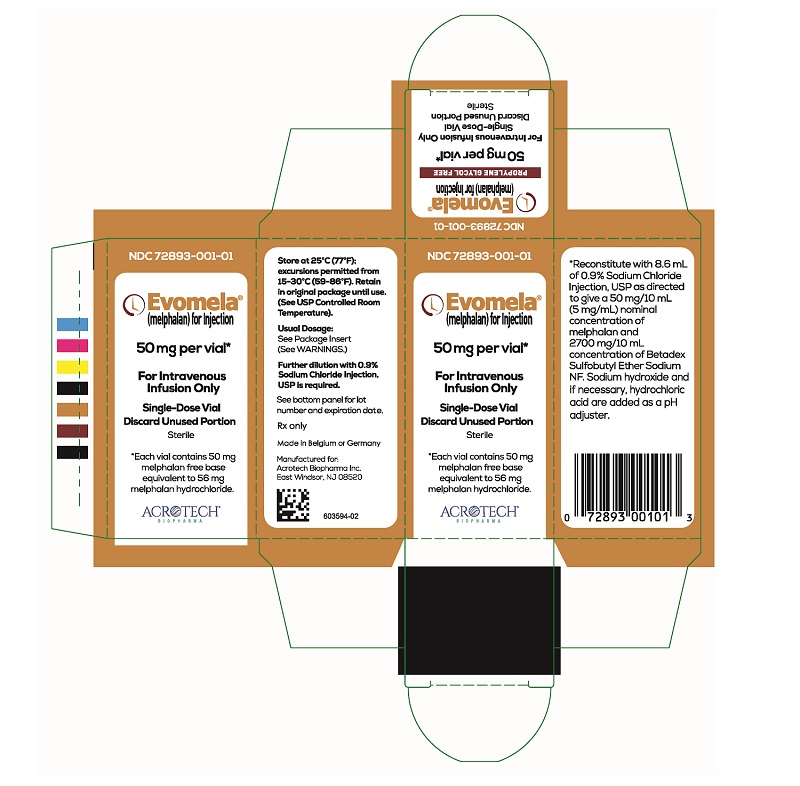
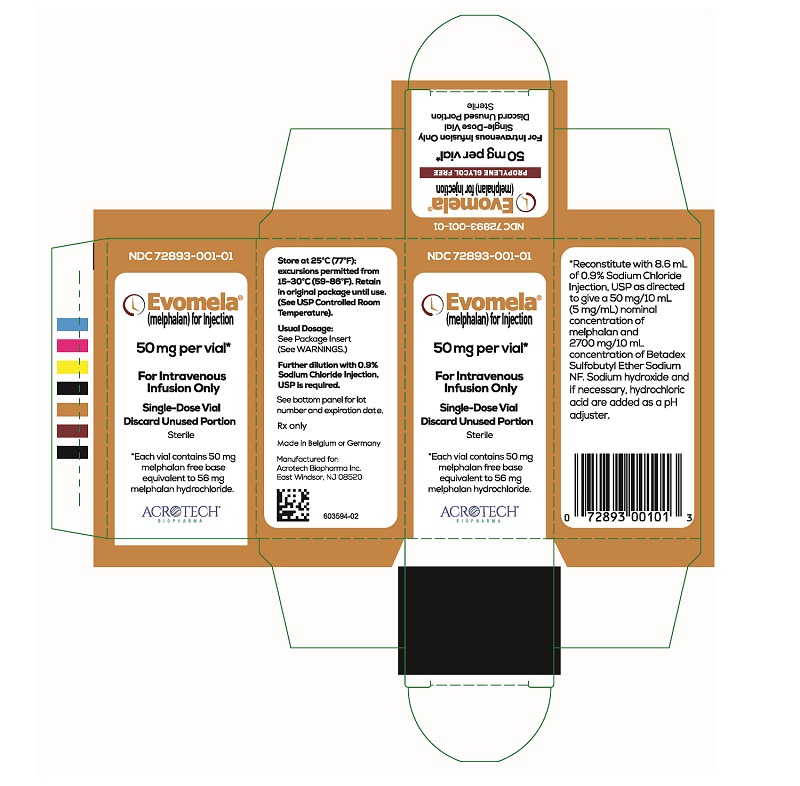
3 DOSAGE FORMS AND STRENGTHSFor injection: 50 mg, white to off-white lyophilized powder in single-dose vial for reconstitution (after reconstitution the solution is clear and colorless to light yellow). Each vial contains 50 ...
-
4 CONTRAINDICATIONSHistory of serious allergic reaction to melphalan.
-
5 WARNINGS AND PRECAUTIONS5.1 Bone Marrow Suppression - For patients receiving Evomela as part of a conditioning regimen, myeloablation occurs in all patients. Do not begin the conditioning regimen if a stem cell product ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described in more detail in other sections of the prescribing information. • Bone Marrow Suppression [see Warnings and Precautions ...
-
7 DRUG INTERACTIONSNo formal drug interaction studies have been conducted. The development of severe renal impairment has been reported in patients treated with a single dose of intravenous melphalan 140-250 mg/m2 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, Evomela can cause fetal harm when administered to a pregnant woman, including teratogenicity and/or embryo-fetal lethality [see ...
-
10 OVERDOSAGEOverdoses resulting in death have been reported with melphalan. Overdoses, including doses up to 290 mg/m2, have produced the following symptoms: severe nausea and vomiting, decreased ...
-
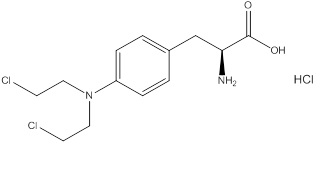
11 DESCRIPTIONEvomela contains melphalan hydrochloride, an alkylating drug, as the active ingredient. The chemical name of melphalan hydrochloride is 4-[bis(2-chloroethyl)amino]-L-phenylalanine hydrochloride ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Melphalan is an alkylating agent of the bischloroethylamine type. As a result, its cytotoxicity appears to be related to the extent of its interstrand cross-linking ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Adequate and well-controlled carcinogenicity studies have not been conducted in animals. However, intraperitoneal (IP) administration ...
-
14. CLINICAL STUDIES14.1 Myeloablative Conditioning in Patients with Multiple Myeloma Undergoing ASCT - An open-label, single-arm, non-randomized trial of Evomela was conducted at 5 US centers (NCT 01660633). The 61 ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. [Accessed on 9 December 2014, from http://www.osha.gov/SLTC/hazardousdrugs/index.html].
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Evomela is supplied in a single carton containing one (1) vial. Each 50 mg vial contains a white to off- white lyophilized powder in single-dose vial for reconstitution (after ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Advise patients or their caregivers of the following: Low Blood Cell Counts - • To report any signs or symptoms ...
-
PATIENT INFORMATIONEVOMELA (ev-o-meh-lah) (melphalan) for injection, for intravenous use - What is Evomela? Evomela is a prescription medicine used in people with a type of cancer called multiple myeloma ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELEvomela Carton Label - NDC 72893-001-01 - Evomela® (melphalan) for Injection - 50 mg per vial* For Intravenous Infusion Only - Single-Use Vial - Discard Unused Portion - Sterile - *Each vial contains 50 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information