Label: ERIVEDGE- vismodegib capsule
- NDC Code(s): 50242-140-01, 50242-140-86
- Packager: Genentech, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ERIVEDGE safely and effectively. See full prescribing information for ERIVEDGE. ERIVEDGE® (vismodegib) capsules, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
- ERIVEDGE can cause embryo-fetal death or severe birth defects when administered to a pregnant woman. ERIVEDGE is embryotoxic, fetotoxic, and teratogenic in animals. Teratogenic effects included severe midline defects, missing digits, and other irreversible malformations [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
- Verify the pregnancy status of females of reproductive potential within 7 days prior to initiating ERIVEDGE. Advise pregnant women of the potential risks to a fetus. Advise females of reproductive potential to use effective contraception during and after ERIVEDGE [see Dosage and Administration (2.1), Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)].
- Advise males of the potential risk of ERIVEDGE exposure through semen and to use condoms with a pregnant partner or a female partner of reproductive potential [see Warnings and Precautions (5.1), Use in Specific Populations (8.3)].
-
1 INDICATIONS AND USAGEERIVEDGE is indicated for the treatment of adults with metastatic basal cell carcinoma, or with locally advanced basal cell carcinoma that has recurred following surgery or who are not candidates ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Safety Information - Verify pregnancy status of females of reproductive potential within 7 days prior to initiating ERIVEDGE [see Use in Specific Populations (8.1 ...
-
3 DOSAGE FORMS AND STRENGTHSCapsules: 150 mg with "150 mg" printed on pink opaque body and "VISMO" printed on grey opaque cap in black ink.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-Fetal Toxicity - Based on its mechanism of action, ERIVEDGE can cause embryo-fetal death or severe birth defects when administered to a pregnant woman. In animal reproduction studies ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Embryo-Fetal Toxicity [see Warnings and Precautions (5.1)] Severe Cutaneous Adverse Reactions [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to ERIVEDGE during pregnancy. Report pregnancies to ...
-
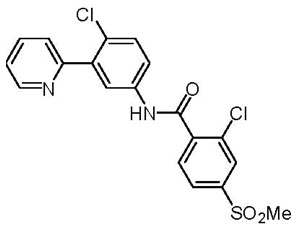
11 DESCRIPTIONVismodegib is a hedgehog (Hh) pathway inhibitor, which is described chemically as 2-Chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(methylsulfonyl)benzamide. The molecular formula is C19H14Cl2N2O3S ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Vismodegib is an inhibitor of the Hedgehog pathway. Vismodegib binds to and inhibits Smoothened, a transmembrane protein involved in Hedgehog signal transduction ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies were performed in mice and rats. No carcinogenic potential was identified in either species. Vismodegib was not ...
-
14 CLINICAL STUDIESA single, international, single-arm, multi-center, open-label, 2-cohort trial [SHH4476g (NCT00833417)] was conducted in 104 patients with either metastatic basal cell carcinoma (mBCC) (n = 33) or ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGERIVEDGE capsules have a pink opaque body and a grey opaque cap with "150 mg" printed on the capsule body and "VISMO" printed on the capsule cap in black ink. ERIVEDGE capsules are available in ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Embryo-Fetal Toxicity [see Warnings and Precautions (5.1), Use in Specific Populations (8.1, 8.3)] Females ...
-
SPL UNCLASSIFIED SECTIONERIVEDGE® (vismodegib) capsules - Manufactured by: Patheon, Inc. Mississauga, Canada - Distributed by: Genentech USA, Inc. A Member of the Roche Group - 1 DNA Way - South San Francisco, CA ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: March 2023 - MEDICATION GUIDE - ERIVEDGE® (EH-rih-vej) (vismodegib) Capsule - What is ...
-
SPL UNCLASSIFIED SECTIONRepresentative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle CartonNDC 50242-140-01 - Erivedge® (vismodegib) capsules - 150 mg - Each capsule contains 150 mg - of vismodegib - Attention Pharmacist: Dispense the accompanying - Medication Guide to each - patient. 28 ...
-
INGREDIENTS AND APPEARANCEProduct Information