Label: VYEPTI- eptinezumab-jjmr injection
- NDC Code(s): 67386-130-51, 67386-130-91
- Packager: Lundbeck Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VYEPTI safely and effectively. See full prescribing information for VYEPTI. VYEPTI® (eptinezumab-jjmr) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVYEPTI is indicated for the preventive treatment of migraine in adults.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - The recommended dosage is 100 mg administered by intravenous infusion every 3 months. Some patients may benefit from a dosage of 300 mg administered by intravenous ...
-
3 DOSAGE FORMS AND STRENGTHSVYEPTI is a clear to slightly opalescent, colorless to brownish-yellow solution available as follows: Injection: 100 mg/mL in a single-dose vial
-
4 CONTRAINDICATIONSVYEPTI is contraindicated in patients with serious hypersensitivity to eptinezumab-jjmr or to any of the excipients in VYEPTI. Reactions have included anaphylaxis and angioedema [see Warnings and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Reactions - Hypersensitivity reactions, including angioedema, urticaria, facial flushing, dyspnea, and rash, have occurred with VYEPTI in clinical trials and in the ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.1)]. Hypertension [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to VYEPTI during pregnancy. Healthcare providers are ...
-
11 DESCRIPTIONEptinezumab-jjmr is a humanized immunoglobulin G1 (IgG1) monoclonal antibody specific for calcitonin gene-related peptide (CGRP) ligand. Eptinezumab-jjmr has an approximate molecular weight of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Eptinezumab-jjmr is a humanized monoclonal antibody that binds to calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor. 12.2 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - The carcinogenic potential of eptinezumab-jjmr has not been assessed. Mutagenesis - Genetic toxicology studies of ...
-
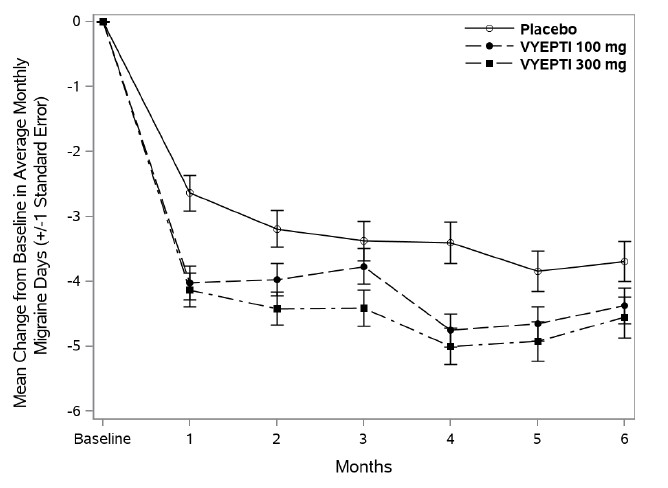
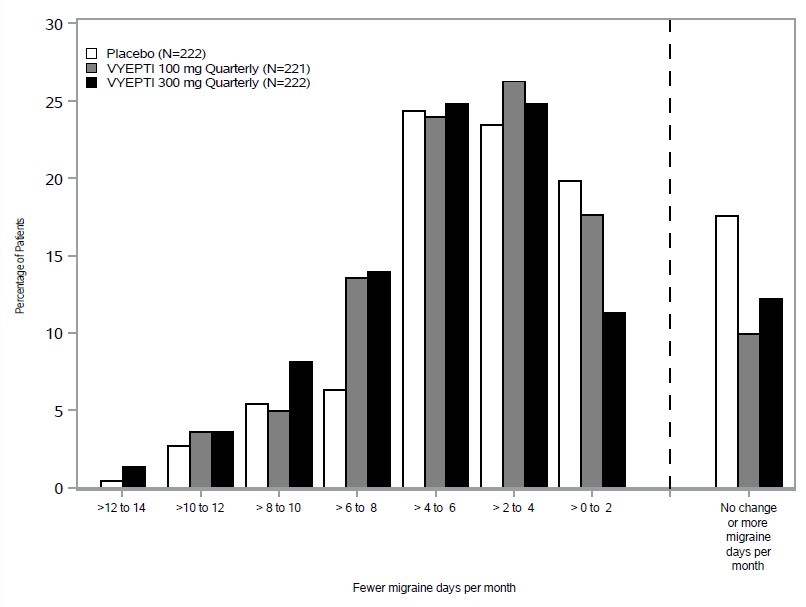
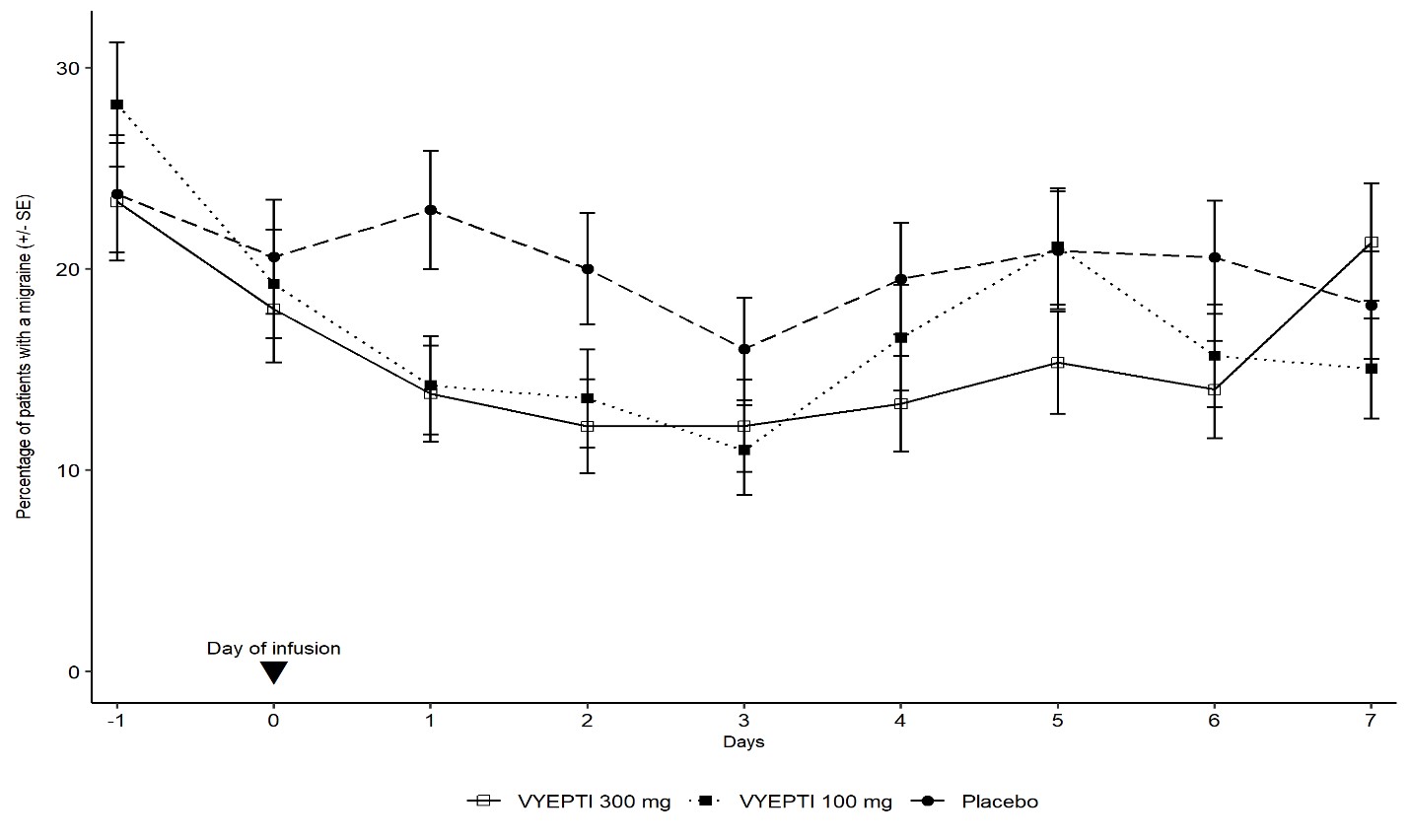
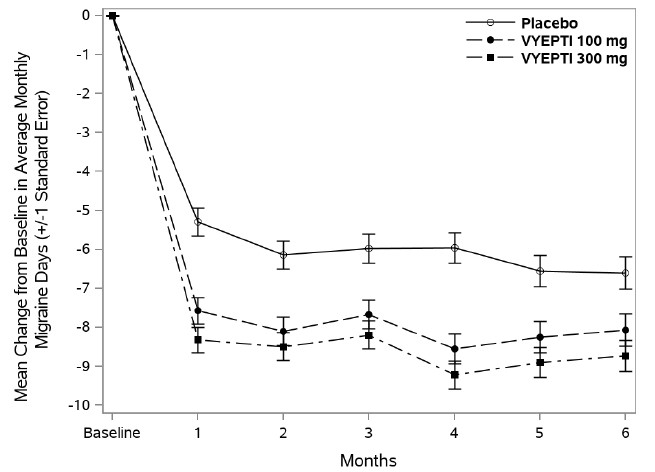
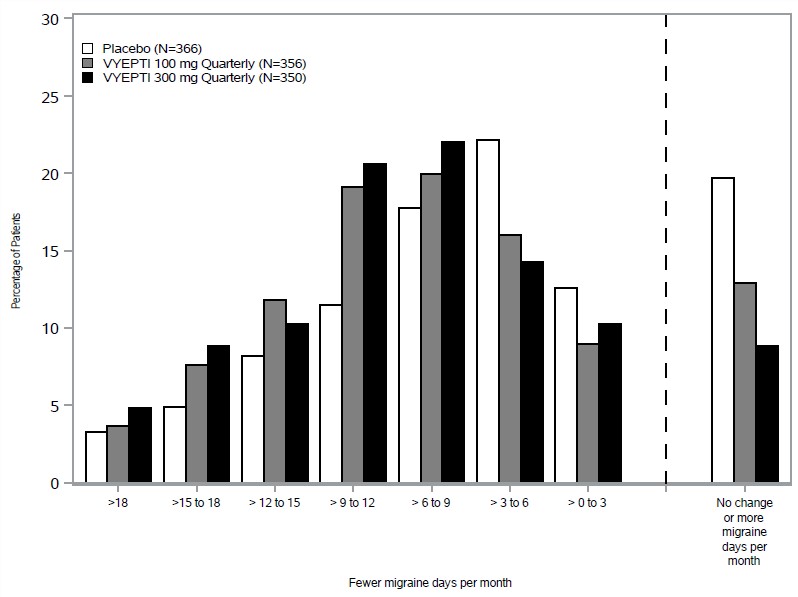
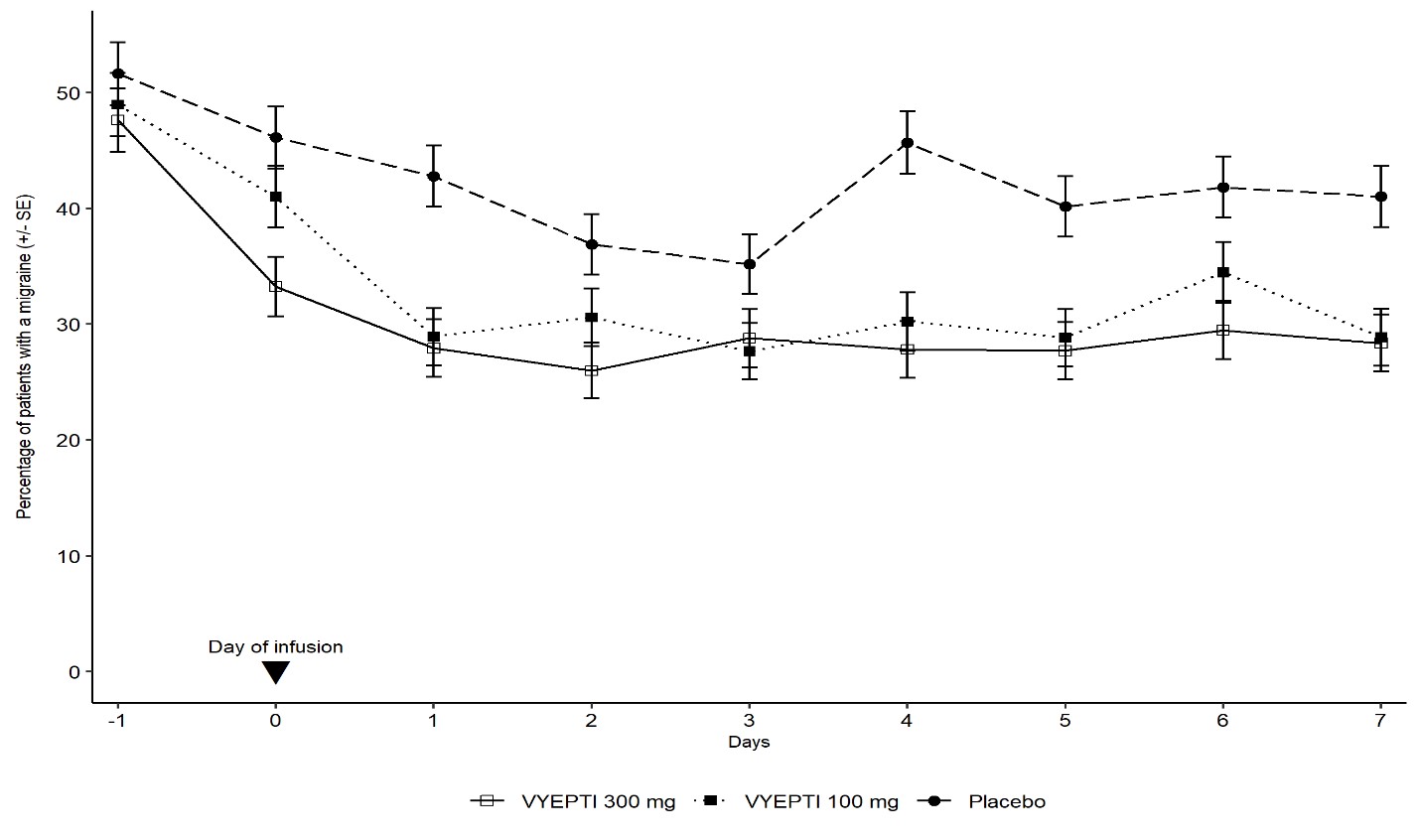
14 CLINICAL STUDIESThe efficacy of VYEPTI was evaluated as a preventive treatment of episodic and chronic migraine in two randomized, multicenter, placebo-controlled studies, both with 6-month double-blind periods ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - VYEPTI (eptinezumab-jjmr) injection is a clear to slightly opalescent, colorless to brownish-yellow solution supplied as: Carton containing one 100 mg/mL single-dose ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Hypersensitivity Reactions - Inform patients about the signs and symptoms of hypersensitivity reactions and that ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - VYEPTI®(vye ep' tee) (eptinezumab-jjmr) injection, for intravenous use - What is VYEPTI? VYEPTI is a prescription medicine used for the preventive treatment of ...
-
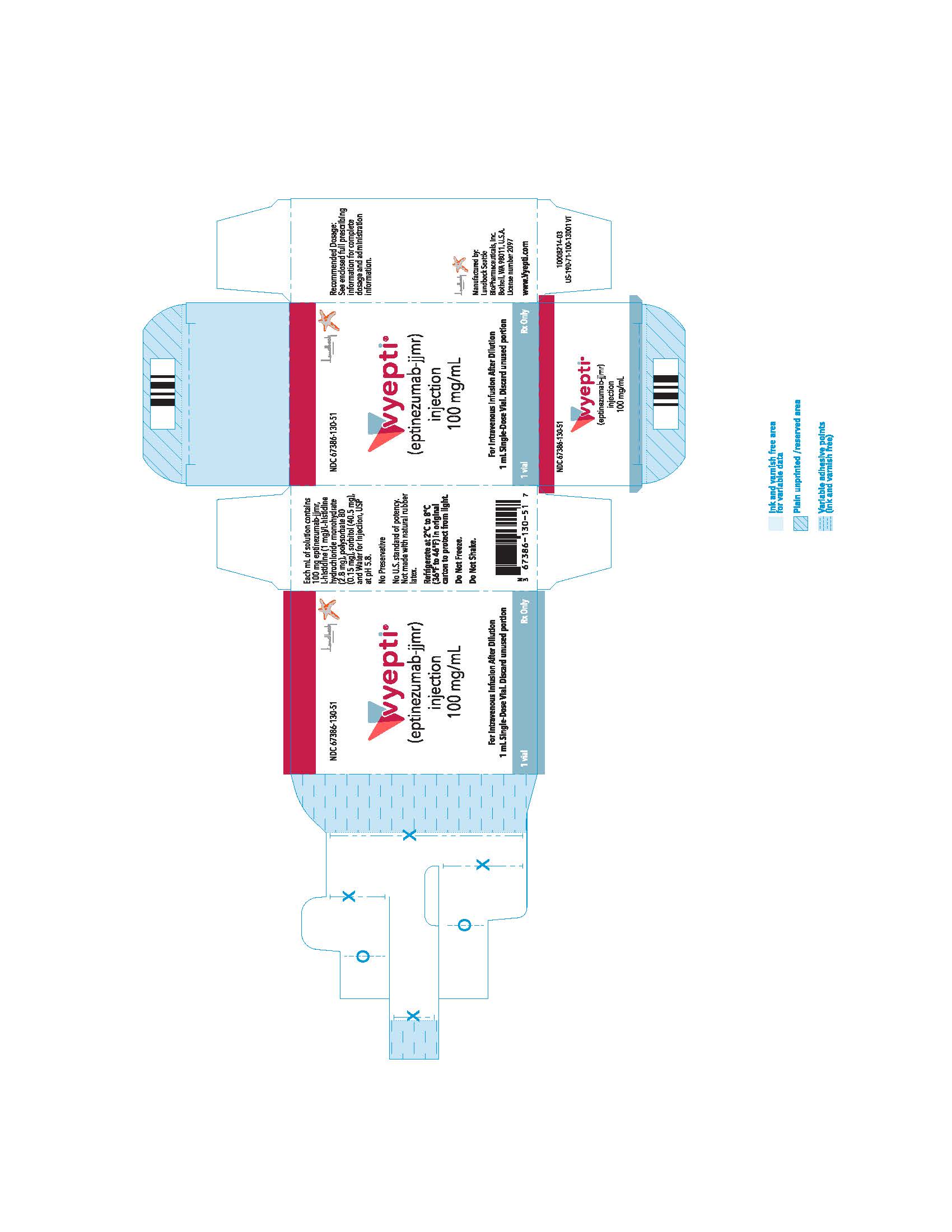
PRINCIPAL DISPLAY PANELNDC 67386-130-51 - VYEPTITM (vye ep' tee) (eptinezumab-jjmr) injection, for intravenous use - 100 mg/mL
-
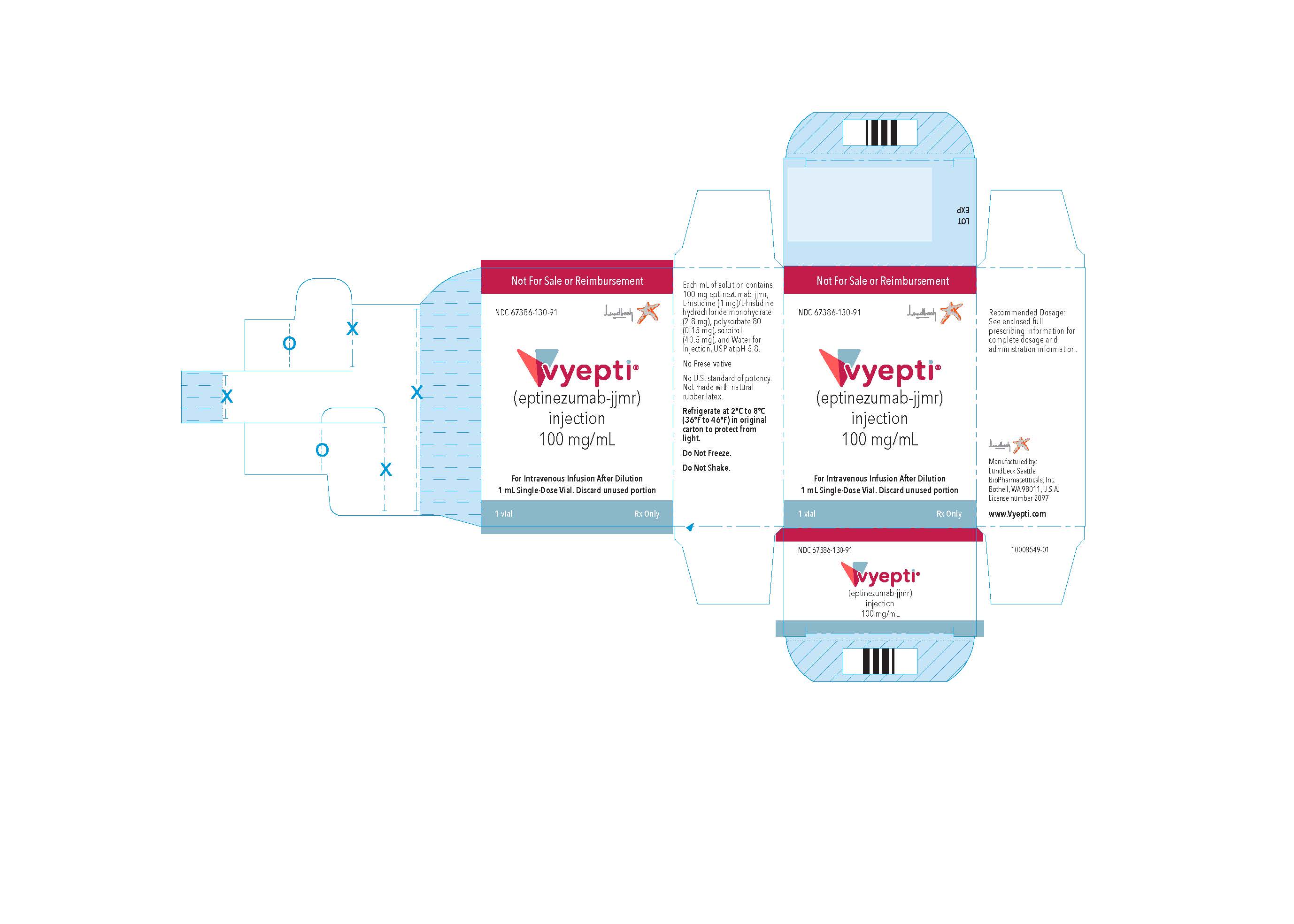
PRINCIPAL DISPLAY PANELNDC 67386-130-91 - VYEPTITM (vye ep' tee) (eptinezumab-jjmr) injection, for intravenous use - 100 mg/mL - Professional Sample - Professional Sample
-
INGREDIENTS AND APPEARANCEProduct Information