Label: ENSTILAR- calcipotriene and betamethasone dipropionate aerosol, foam
- NDC Code(s): 50222-302-60, 50222-302-66, 50222-302-91
- Packager: LEO Pharma Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ENSTILAR Foam safely and effectively. See full prescribing information for ENSTILAR Foam. ENSTILAR® (calcipotriene and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEEnstilar® (calcipotriene and betamethasone dipropionate) Foam is indicated for the topical treatment of plaque psoriasis in patients 12 years and older.
-
2 DOSAGE AND ADMINISTRATIONShake can prior to using Enstilar Foam. Apply Enstilar Foam to affected areas once daily for up to 4 weeks. The maximum dose should not exceed 60 grams every 4 days. Rub in Enstilar Foam gently ...
-
3 DOSAGE FORMS AND STRENGTHSEnstilar Foam: 0.005%/0.064% - each gram contains 50 mcg calcipotriene and 0.643 mg of betamethasone dipropionate in a white to off-white opalescent liquid in a pressurized aluminum spray can with ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Flammability - The propellants in Enstilar Foam are flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. 5.2 Hypercalcemia and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data with Enstilar Foam are not sufficient to evaluate a drug-associated risk for major birth defects, miscarriages, or adverse maternal or fetal ...
-
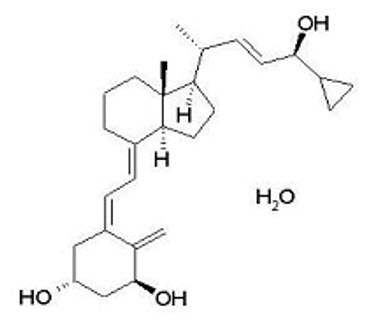
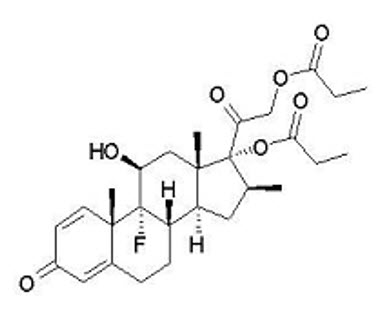
11 DESCRIPTIONEnstilar Foam contains calcipotriene hydrate and betamethasone dipropionate. It is for topical use only. Calcipotriene Hydrate - Calcipotriene hydrate is a synthetic vitamin D analog and has ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Enstilar Foam combines the pharmacological effects of calcipotriene hydrate as a synthetic vitamin D3 analog and betamethasone dipropionate as a synthetic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - When calcipotriene was applied topically to mice for up to 24 months at dosages of 3, 10, and 30 mcg/kg/day (9, 30, and 90 mcg/m2/day ...
-
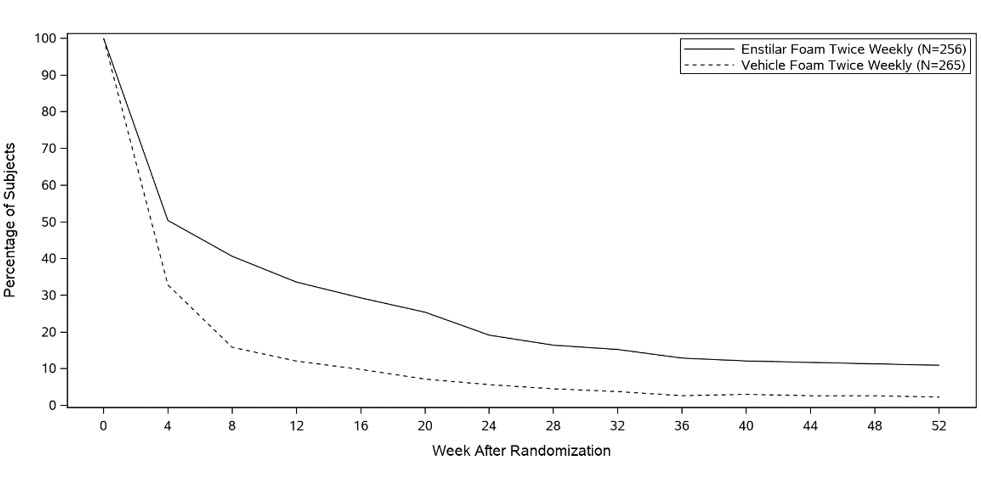
14 CLINICAL STUDIESTwo multicenter, randomized, double-blind trials were conducted in adult subjects with plaque psoriasis. In Trial One, 302 subjects were randomized to 1 of 3 treatment groups: Enstilar® Foam ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Enstilar (calcipotriene and betamethasone dipropionate) Foam, 0.005%/0.064% is a white to off-white opalescent liquid in a pressurized aluminum spray can with a continuous ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information and Instructions for Use). Flammability - Instruct patients that Enstilar Foam is flammable; avoid heat, flame, or smoking when applying ...
-
SPL UNCLASSIFIED SECTIONManufactured by: LEO Laboratories Ltd., 285 Cashel Road, Dublin 12, Ireland - Distributed by: LEO Pharma Inc., Madison, NJ 07940, USA - Enstilar® is a registered trademark of LEO Pharma A/S. © 2022 ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - ENSTILAR® [EN-still-ar] (calcipotriene and betamethasone dipropionate) Foam - This Patient Information has been approved by the U.S. Food and Drug ...
-
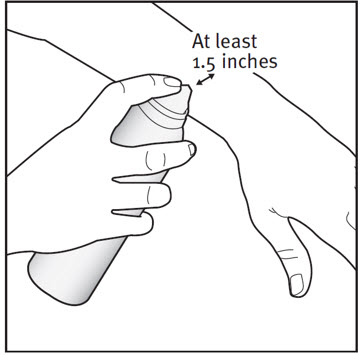
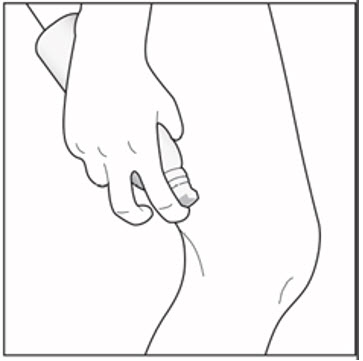
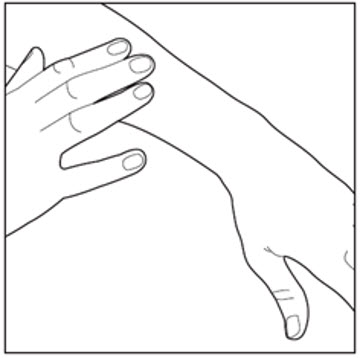
INSTRUCTIONS FOR USE ENSTILAR® [EN-still-ar] (calcipotriene and betamethasone dipropionate)FoamThis Instructions for Use contains information on how to apply Enstilar Foam. Important Information You Need to Know Before Applying Enstilar Foam: Enstilar Foam is for use on skin only ...
-
PRINCIPAL DISPLAY PANEL - 60 gram Can CartonNDC 50222-302-60 - Rx only - Enstilar® (calcipotriene and - betamethasone - dipropionate) Foam, 0.005%/0.064% Shake before Use - For Topical Use Only - Not for oral, ophthalmic, or intravaginal use - Net ...
-
PRINCIPAL DISPLAY PANEL - 120 gram Can CartonNDC 50222-302-66 - Rx only - Enstilar® (calcipotriene and betamethasone - dipropionate) Foam, 0.005%/0.064% Shake before Use - For Topical Use Only - Not for oral, ophthalmic, or intravaginal use - Net ...
-
INGREDIENTS AND APPEARANCEProduct Information