Label: ENDOMETRIN- progesterone insert
- NDC Code(s): 55566-6500-1, 55566-6500-2, 55566-6500-3
- Packager: Ferring Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 16, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ENDOMETRIN safely and effectively. See full prescribing information for ENDOMETRIN. ENDOMETRIN ® (progesterone) Vaginal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEENDOMETRIN® (progesterone) is indicated to support embryo implantation and early pregnancy by supplementation of corpus luteal function as part of an Assisted Reproductive Technology (ART ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - The dose of ENDOMETRIN is 100 mg administered vaginally two or three times daily starting the day after oocyte retrieval and continuing for up to 10 weeks total ...
-
3 DOSAGE FORMS AND STRENGTHS100 mg vaginal insert is a white to off-white oblong-shaped tablet debossed with "FPI" on one side and "100" on the other side.
-
4 CONTRAINDICATIONSENDOMETRIN is contraindicated in individuals with any of the following conditions: Previous allergic reactions to progesterone or any of the ingredients of ENDOMETRIN [see Description ...
-
5 WARNINGS AND PRECAUTIONS5.1 Cardiovascular or Cerebrovascular Disorders - The physician should be alert to earliest signs of myocardial infarction, cerebrovascular disorders, arterial or venous thromboembolism (venous ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo formal drug-drug interaction studies have been conducted for ENDOMETRIN. Drugs known to induce the hepatic cytochrome-P450-3A4 system (such as rifampin, carbamazepine) may increase the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - ENDOMETRIN has been used to support embryo implantation and maintain clinical pregnancy in one clinical study. The live birth outcomes of these pregnancies were as follows: Among ...
-
10 OVERDOSAGETreatment of overdosage consists of discontinuation of ENDOMETRIN together with institution of appropriate symptomatic and supportive care.
-
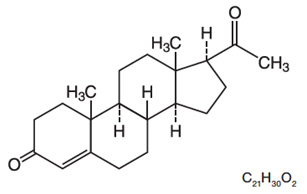
11 DESCRIPTIONENDOMETRIN (progesterone) Vaginal Insert contains micronized progesterone. ENDOMETRIN is supplied with polyethylene vaginal applicators. The active ingredient, progesterone, is present in 100 mg ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Progesterone is a naturally occurring steroid that is secreted by the ovary, placenta, and adrenal gland. In the presence of adequate estrogen, progesterone transforms ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Nonclinical toxicity studies to determine the potential of ENDOMETRIN to cause carcinogenicity or mutagenicity have not been performed ...
-
14 CLINICAL STUDIES14.1 Luteal Supplementation During Assisted Reproductive Treatment Study - A randomized, open-label, active-controlled study evaluated the efficacy of 10 weeks of treatment with two different ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach ENDOMETRIN Vaginal Insert is a white to off-white oblong-shaped insert debossed with "FPI" on one side and "100" on the other side. Each ENDOMETRIN (progesterone) Vaginal Insert, 100 mg, is ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (17.4). 17.1 Vaginal Bleeding - Inform patients of the importance of reporting irregular vaginal bleeding to their doctor as soon as possible. 17.2 Common ...
-
PATIENT PACKAGE INSERTIMPORTANT: For Vaginal Use Only. Read the patient information that comes with ENDOMETRIN ® (progesterone) before you start to use it and each time you get a refill. There may be new information ...
-
PRINCIPAL DISPLAY PANEL - 100 mg CartonNDC 55566-6500-3 - Endometrin® (progesterone) Vaginal Insert 100mg - Contents: 21 vaginal inserts with 21 disposable vaginal applicators - Each insert contains 100 mg progesterone, USP - FOR VAGINAL USE ...
-
INGREDIENTS AND APPEARANCEProduct Information