Label: ENDARI- glutamine powder, for solution
- NDC Code(s): 42457-420-01, 42457-420-60
- Packager: Emmaus Medical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ENDARI™ safely and effectively. See full prescribing information for ENDARI. ENDARI (L-glutamine oral powder ) Initial U.S ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEEndari is indicated to reduce the acute complications of sickle cell disease in adult and pediatric patients 5 years of age and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Dosage - Administer Endari orally, twice per day at the dose based on body weight according to Table 1. Table 1. Recommended Dosing - Weight in kilogramsWeight in poundsPer dose in ...
-
3 DOSAGE FORMS AND STRENGTHSOral powder: 5 grams of L–glutamine as a white crystalline powder in paper-foil-plastic laminate packets
-
4 CONTRAINDICATIONSNone
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on Endari use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. Animal reproduction ...
-
10 OVERDOSAGESingle oral doses of L-glutamine at about 20 g/kg to 22 g/kg, 8 g/kg to 11 g/kg, and 19 g/kg were lethal in mice, rats, and rabbits, respectively. Supportive measures should be undertaken in the ...
-
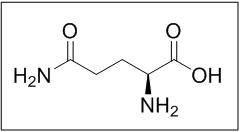
11 DESCRIPTIONEndari (L-glutamine) is an amino acid. L-glutamine is designated chemically as (S)-2-aminoglutaramic acid, L-glutamic acid 5-amide, or (S)-2,5-diamino-5-oxopentanoic acid. The molecular formula ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of the amino acid L-glutamine in treating sickle cell disease (SCD) is not fully understood. Oxidative stress phenomena are involved in the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate the carcinogenic potential of L-glutamine. L-glutamine was not ...
-
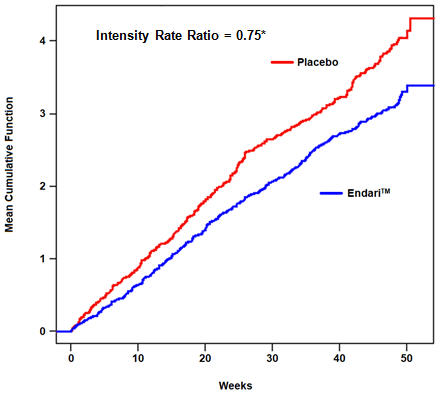
14 Clinical StudiesThe efficacy of Endari in sickle cell disease was evaluated in a randomized, double-blind, placebo-controlled, multi-center clinical trial entitled "A Phase III Safety and Efficacy Study of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEndari is supplied in paper-foil-plastic laminate packets containing 5 grams of L-glutamine white crystalline powder. Carton of 60 packets: NDC 42457-420-60 - Store at 20°C to 25°C (68°F to ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Dosage and Administration - Advise patient to take a missed dose as soon as they remember. Patient should ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Emmaus Medical, Inc - Torrance, CA 90503
-
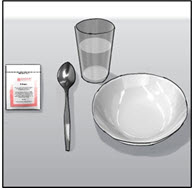
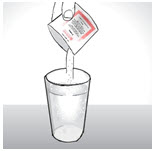
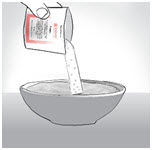
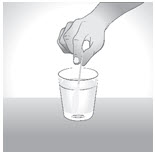
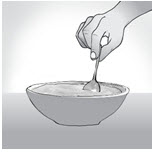
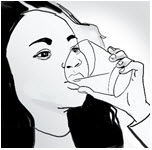
INSTRUCTIONS FOR USEENDARI® (en-dar-ee) (L-glutamine oral powder) Read this Instructions for Use before you start taking Endari and each time you get a refill. There may be new information. This Instructions for Use ...
-
PRINCIPAL DISPLAY PANEL - 5 gram Packet CartonNDC: 42457-420-60 - ENDARI™ (L-glutamine oral powder) Contents: 60 Packets (5 grams/packet) Directions: Mix the contents of each packet with cold or room temperature beverage or ...
-
INGREDIENTS AND APPEARANCEProduct Information