Label: IDHIFA- enasidenib mesylate tablet, film coated
- NDC Code(s): 59572-705-30, 59572-710-30
- Packager: Celgene Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use IDHIFA safely and effectively. See full prescribing information for IDHIFA. IDHIFA® (enasidenib) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: DIFFERENTIATION SYNDROME
Patients treated with IDHIFA have experienced symptoms of differentiation syndrome, which can be fatal if not treated. Symptoms may include fever, dyspnea, acute respiratory distress, pulmonary infiltrates, pleural or pericardial effusions, rapid weight gain or peripheral edema, lymphadenopathy, bone pain, and hepatic, renal, or multi-organ dysfunction. If differentiation syndrome is suspected, initiate corticosteroid therapy and hemodynamic monitoring until symptom resolution [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Close -
1 INDICATIONS AND USAGE1.1 Acute Myeloid Leukemia - IDHIFA is indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation as ...
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - Select patients for the treatment of AML with IDHIFA based on the presence of IDH2 mutations in the blood or bone marrow [see Indications and Usage (1.1) and Clinical ...
-
3 DOSAGE FORMS AND STRENGTHSIDHIFA is available in the following tablet strengths: • 50 mg enasidenib tablet: Pale yellow to yellow oval-shaped film-coated tablet debossed "ENA" on one side and "50" on the other side. • 100 ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Differentiation Syndrome - In the clinical trial, 14% of patients treated with IDHIFA experienced differentiation syndrome, which may be life-threatening or fatal if not treated ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Differentiation Syndrome [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Effect of IDHIFA on Other Drugs - Certain CYP1A2 Substrates - Avoid concomitant use with IDHIFA unless otherwise recommended in the Prescribing Information for CYP1A2 substrates where ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal embryo-fetal toxicity studies, IDHIFA can cause fetal harm when administered to a pregnant woman. There are no available data on IDHIFA use in ...
-
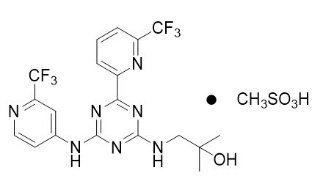
11 DESCRIPTIONEnasidenib is an inhibitor of isocitrate dehydrogenase-2 (IDH2) enzyme. Enasidenib is available as the mesylate salt with the chemical ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Enasidenib is a small molecule inhibitor of the isocitrate dehydrogenase 2 (IDH2) enzyme. Enasidenib targets the mutant IDH2 variants R140Q, R172S, and R172K at ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been performed with enasidenib. Enasidenib was not mutagenic in an in vitro bacterial reverse mutation ...
-
14 CLINICAL STUDIES14.1 Acute Myeloid Leukemia - The efficacy of IDHIFA was evaluated in an open-label, single-arm, multicenter, two-cohort clinical trial (Study AG221-C-001, NCT01915498) of 199 adult patients with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - 50 mg tablet: Pale yellow to yellow oval-shaped film-coated tablet debossed “ENA” on one side and “50” on the other side. • 30-count bottles of 50 mg tablets with a desiccant ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Differentiation Syndrome - Advise patients on the risks of developing differentiation syndrome as early as 1 day ...
-
MEDICATION GUIDEMEDICATION GUIDE - IDHIFA® (eyed-HEE-fuh) (enasidenib) tablets - What is the most important information I should know about IDHIFA? IDHIFA may cause serious side effects ...
-
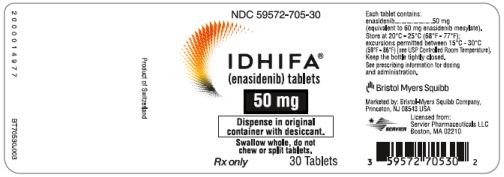
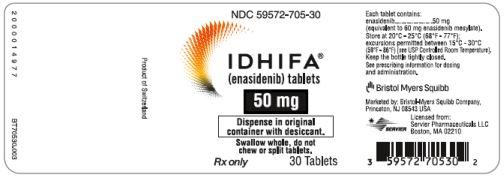
PRINCIPAL DISPLAY PANEL - 50 mg Tablet Bottle LabelNDC 59572-705-30 - IDHIFA® (enasidenib) tablets - 50 mg - Dispense in original - container with desiccant. Swallow whole, do not - chew or split tablets. Rx only - 30 Tablets
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 59572-710-30 - IDHIFA® (enasidenib) tablets - 100 mg - Dispense in original - container with desiccant. Swallow whole, do not - chew or split tablets. Rx only - 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information