Label: ENABLEX- darifenacin tablet, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-5363-0, 54868-5704-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0078-0419, 0078-0420
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 1, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
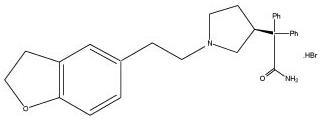
ENABLEX® (darifenacin) is an extended-release tablet which contains 7.5 mg or 15 mg darifenacin as its hydrobromide salt. The active moiety, darifenacin, is a potent muscarinic receptor ...
-
CLINICAL PHARMACOLOGY
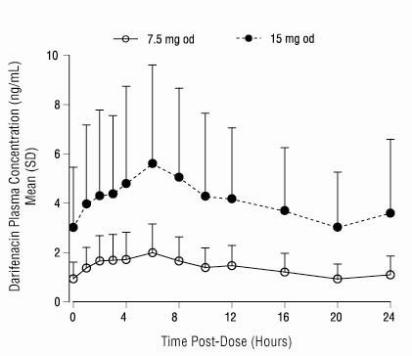
General - Darifenacin is a competitive muscarinic receptor antagonist. Muscarinic receptors play an important role in several major cholinergically mediated functions, including contractions of ...
-
CLINICAL STUDIES
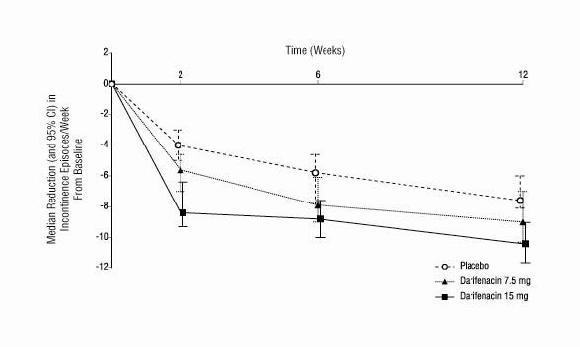
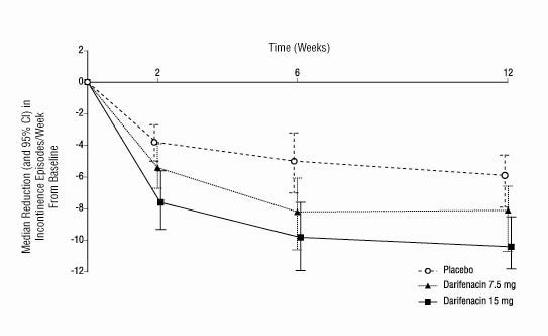
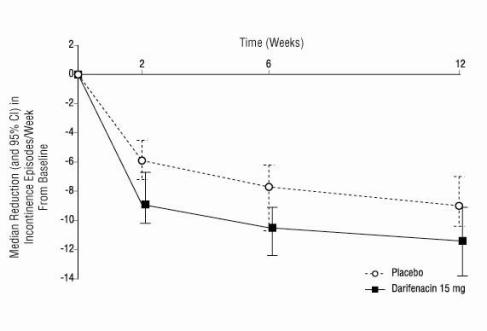
ENABLEX® (darifenacin) extended-release tablets were evaluated for the treatment of patients with overactive bladder with symptoms of urgency, urge urinary incontinence, and increased urinary ...
-
INDICATIONS AND USAGE
ENABLEX® (darifenacin) extended-release tablets are indicated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency and frequency.
-
CONTRAINDICATIONS
ENABLEX® (darifenacin) extended-release tablets are contraindicated in patients with urinary retention, gastric retention or uncontrolled narrow-angle glaucoma and in patients who are at risk for ...
-
PRECAUTIONS
General - Risk of Urinary Retention - ENABLEX® (darifenacin) extended-release tablets should be administered with caution to patients with clinically significant bladder outflow obstruction ...
-
ADVERSE REACTIONS
During the clinical development of ENABLEX® (darifenacin) extended-release tablets, a total of 7,363 patients and volunteers were treated with doses of darifenacin from 3.75 mg to 75 mg once ...
-
OVERDOSAGE
Overdosage with antimuscarinic agents, including ENABLEX® (darifenacin) extended-release tablets, can result in severe antimuscarinic effects. Treatment should be symptomatic and supportive. In ...
-
DOSAGE AND ADMINISTRATION
The recommended starting dose of ENABLEX® (darifenacin) extended-release tablets is 7.5 mg once daily. Based upon individual response, the dose may be increased to 15 mg once daily, as early as ...
-
HOW SUPPLIED
ENABLEX® 7.5 mg extended-release tablets are round, shallow, convex, white-colored tablets, and are identified with “DF” on one side and “7.5” on the reverse. Bottle of 30 - NDC ...
-
INFORMATION FOR PATIENTS
ENABLEX® (ěn-ā-blěx) (darifenacin) Extended-release tablets - 7.5 mg or 15 mg - Rx only - Read the Patient Information that comes with ENABLEX® before you start taking it and each time you ...
-
PRINCIPAL DISPLAY PANEL
Package Label – 7.5 mg per tablet - Rx Only - Enablex® (darifenacin) Extended-release tablets
-
PRINCIPAL DISPLAY PANEL
Package Label – 15 mg per tablet - Rx Only - Enablex® (darifenacin) Extended-release tablets
-
INGREDIENTS AND APPEARANCEProduct Information