Label: EGRIFTA SV- tesamorelin kit
- NDC Code(s): 62064-241-30
- Packager: Theratechnologies Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EGRIFTA SV safely and effectively. See full prescribing information for EGRIFTA SV. EGRIFTA SV® (tesamorelin) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
EGRIFTA SV is indicated for the reduction of excess abdominal fat in HIV-infected adult patients with lipodystrophy. Limitations of Use: Long-term cardiovascular safety of EGRIFTA SV has not been ...
-
2 DOSAGE AND ADMINISTRATION
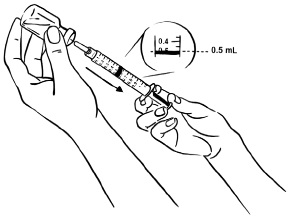
2.1 Dosage and Administration - • The dosage and administration recommendations in this prescribing information only apply to EGRIFTA SV (tesamorelin) for injection 2 mg per vial formulation. For ...
-
3 DOSAGE FORMS AND STRENGTHS
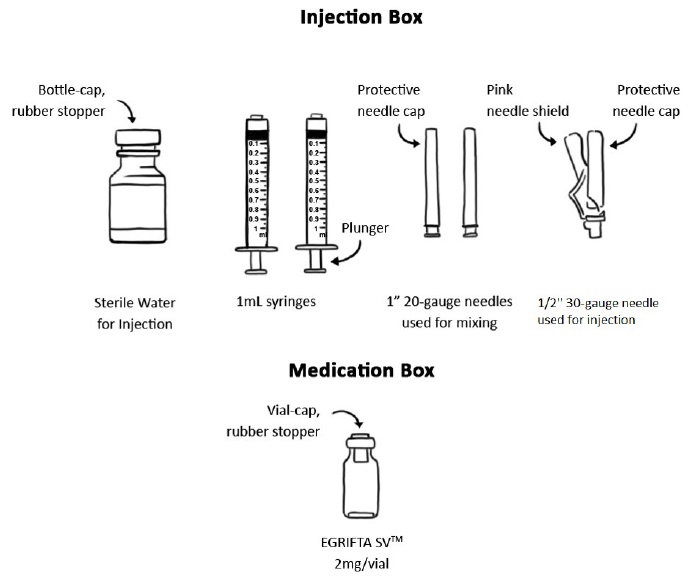
For injection: 2 mg of tesamorelin as a white to off-white lyophilized powder in a single-dose vial and a diluent of 10 mL of Sterile Water for Injection.
-
4 CONTRAINDICATIONS
EGRIFTA SV is contraindicated in: Patients with disruption of the hypothalamic-pituitary axis due to hypophysectomy, hypopituitarism, pituitary tumor/surgery, head irradiation or head ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Neoplasms - New Malignancy - Carefully consider the decision to start treatment with EGRIFTA SV based on the increased background risk of malignancies in HIV-positive ...
-
6 ADVERSE REACTIONS
The following important adverse reactions are also described elsewhere in the labeling: Increased risk of neoplasms [see Warnings and Precautions (5.1)] Elevated IGF-1 levels [see Warnings and ...
-
7 DRUG INTERACTIONS
7.1 Cytochrome P450-Metabolized Drugs - Co-administration of tesamorelin with simvastatin, a CYP3A substrate had no significant impact on the pharmacokinetics profiles of simvastatin in healthy ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - EGRIFTA SV is contraindicated in pregnant women because modifying visceral adipose tissue offers no benefit in pregnant women and could result in fetal harm [see ...
-
11 DESCRIPTION
Tesamorelin is a human growth hormone-releasing factor (GRF) analog produced synthetically. It is comprised of the 44 amino acid sequence of human GRF and a hexenoyl moiety, a C6 chain with a ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - In vitro, tesamorelin binds and stimulates human GRF receptors with similar potency as the endogenous GRF [see Clinical Pharmacology (12.2)]. Growth hormone-releasing ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Life-time carcinogenicity studies in rodents have not been conducted with tesamorelin acetate. No potential mutagenicity of ...
-
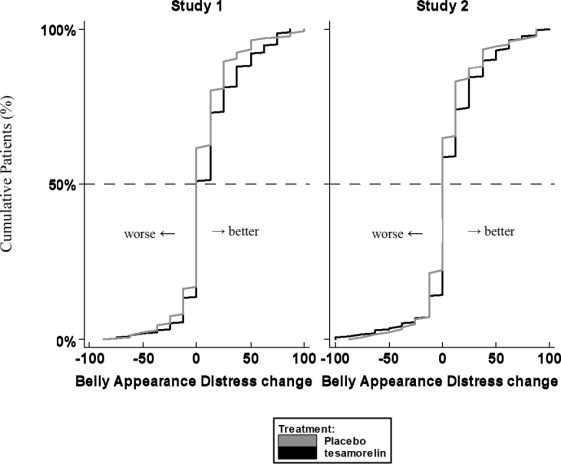
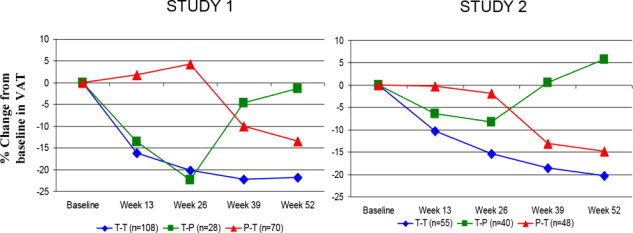
14 CLINICAL STUDIES
The safety and effectiveness of EGRIFTA SV (2 mg/vial formulation) has been established based on adequate and well controlled studies with EGRIFTA (1 mg/vial formulation), as well as a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
EGRIFTA SV (tesamorelin) for injection is supplied as a white to off-white lyophilized powder in a 2 mg single-dose vial with a diluent of 10 mL vial of Sterile Water for Injection. EGRIFTA SV ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Increased Risk of Malignancy - Inform patients about the increased background risk of ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - EGRIFTA SV® (eh-GRIF-tuh ESS-vee) (tesamorelin) for injection - for subcutaneous use - 2 mg vial - Read the Patient Information that comes with EGRIFTA SV before you start ...
-
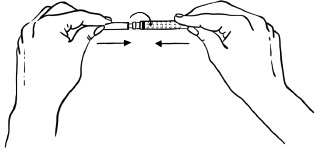
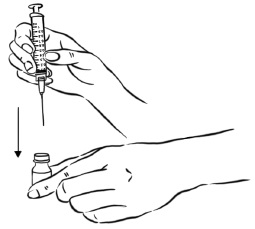
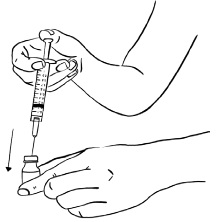
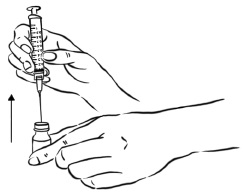
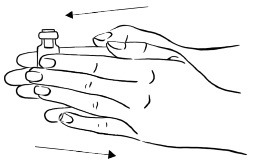
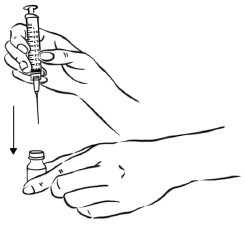
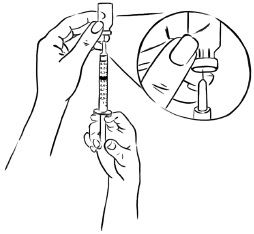
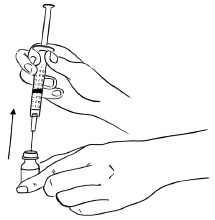
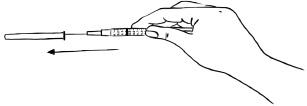
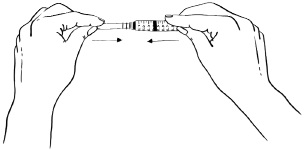
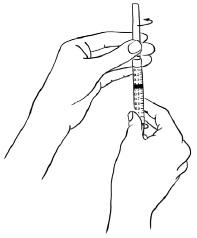
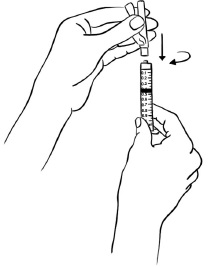
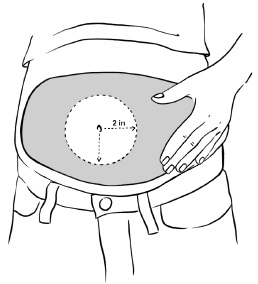
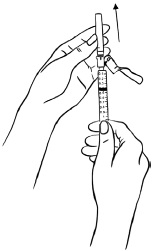
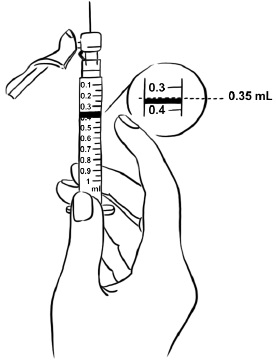
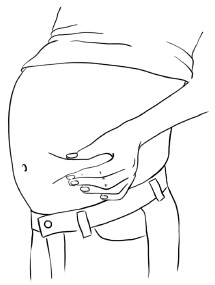
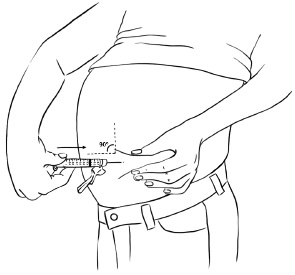
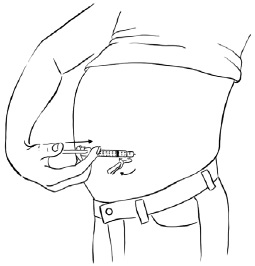
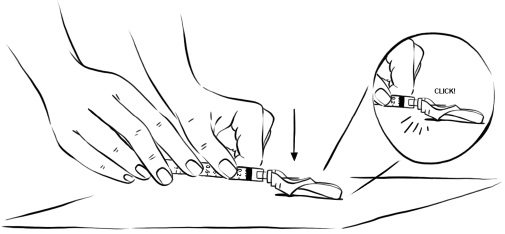
INSTRUCTIONS FOR USEInstructions for Use - EGRIFTA SV® (eh-GRIF-tuh ESS-vee) (tesamorelin) for injection - for subcutaneous use - This Instructions for Use contains step-by-step information on how to use the 2 mg vial ...
-
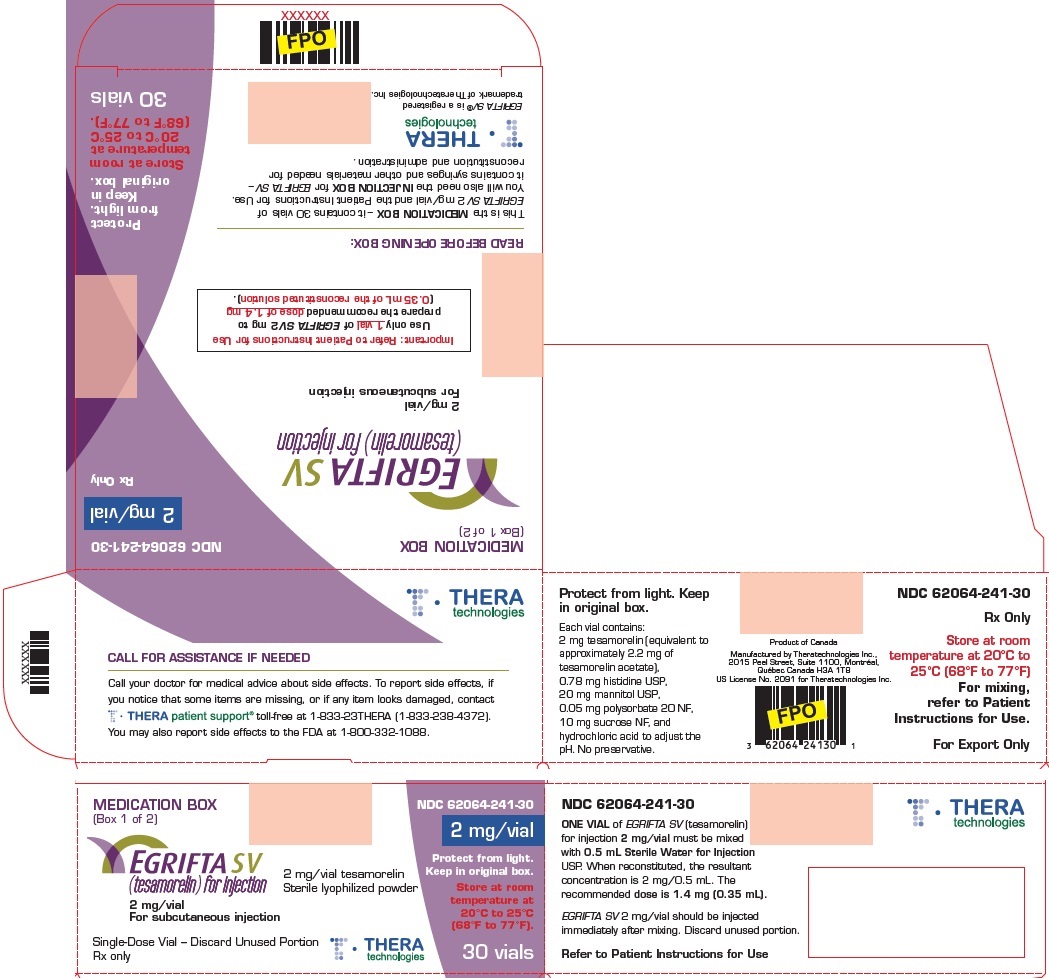
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Medication Box - MEDICATION BOX - (Box 1 of 2) NDC 62064-241-30 - 2 mg/vial - Rx only - EGRIFTA SV® (tesamorelin) for injection - 2 mg/vial - For subcutaneous injection ...
-
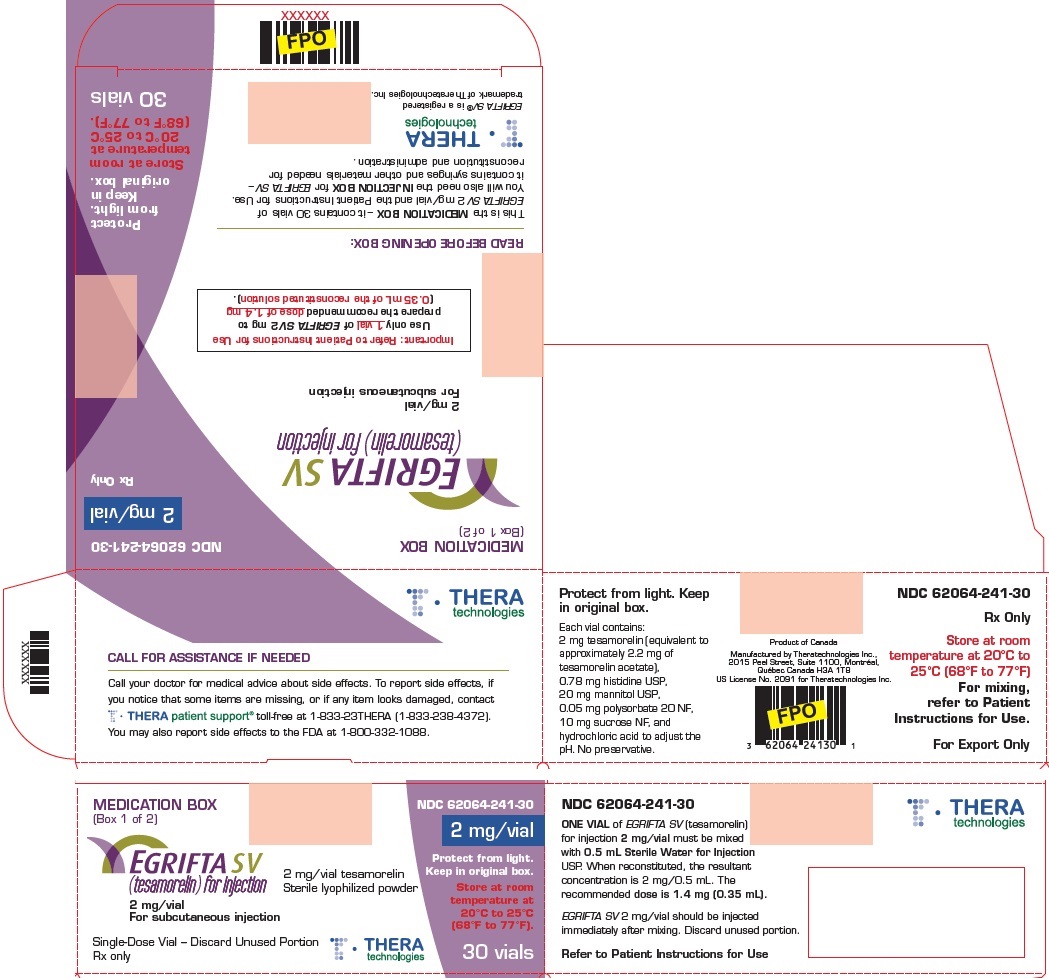
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Injection Box - INJECTION BOX (Box 2 of 2) For use only with EGRIFTA SV® 2 mg/vial - (tesamorelin) for injection 2 mg/vial - For mixing, refer to Patient - Instructions ...
-
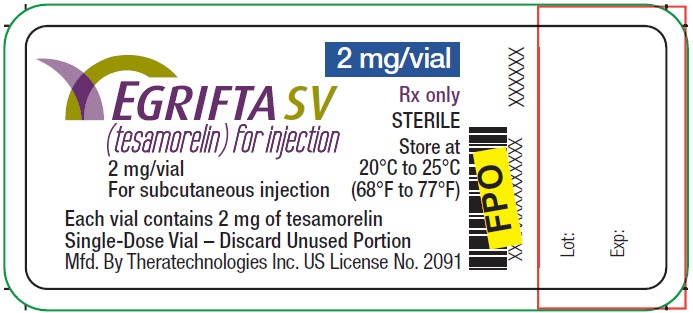
PRINCIPAL DISPLAY PANELPrincipal Display Panel - 2 mg Vial Label - 2 mg/vial - EGRIFTA SV® (tesamorelin) for injection - Rx only - STERILE - Store at - 20°C to 25°C - (68° to 77°F) For subcutaneous injection ...
-
INGREDIENTS AND APPEARANCEProduct Information





 toll-free at 1-833-23THERA (1-833-238-4372).
toll-free at 1-833-23THERA (1-833-238-4372).