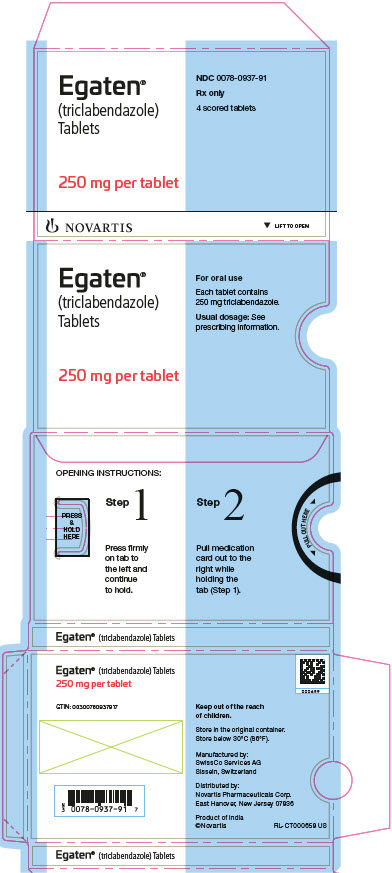
Label: EGATEN- triclabendazole tablet
- NDC Code(s): 0078-0937-91
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 5, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use EGATEN safely and effectively. See full prescribing information for EGATEN. EGATEN® (triclabendazole) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
EGATEN® is indicated for the treatment of fascioliasis in patients 6 years of age and older.
-
2
DOSAGE AND ADMINISTRATION
The recommended dose of EGATEN is 2 doses of 10 mg/kg given 12 hours apart in patients 6 years of age and older. The 250 mg tablets are functionally scored and divisible into two equal halves of ...
-
3
DOSAGE FORMS AND STRENGTHS
EGATEN (triclabendazole) tablet: 250 mg pale red, speckled, capsule shaped, biconvex with “EG ⅁Ǝ” debossed on one side and functionally scored on both sides.
-
4
CONTRAINDICATIONS
EGATEN is contraindicated in patients with known hypersensitivity to triclabendazole and/or to other benzimidazole derivatives or to any of the excipients in EGATEN.
-
5
WARNINGS AND PRECAUTIONS
5.1 - QT Prolongation - EGATEN prolongs the QTc interval [see Clinical Pharmacology (12.2)]. The magnitude of QTc prolongation can increase with increasing treatment duration of EGATEN ...
-
6
ADVERSE REACTIONS
6.1 - Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be ...
-
7
DRUG INTERACTIONS
7.1 - Effect of EGATEN on CYP2C19 Substrates - No specific clinical drug interaction studies have been conducted for triclabendazole. However, in vitro data suggest the potential for ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no available data on EGATEN use in pregnant women to inform a drug-associated risk of major birth defects, miscarriage or adverse maternal or ...
-
10
OVERDOSAGE
The reported symptom of overdosage following ingestion of approximately 54 mg/kg of EGATEN (approximately 2.7 times the recommended dose) was nausea. The patient recovered following osmotic ...
-
11
DESCRIPTION
EGATEN (triclabendazole) tablet is an orally administered anthelmintic for immediate release. Triclabendazole is designated chemically as benzimidazole derivative, 6-chloro-5-(2 ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Triclabendazole is an anthelmintic against Fasciola species [see Microbiology (12.4)]. 12.2 - Pharmacodynamics - Triclabendazole exposure-response ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Mutagenesis - No genotoxic potential was noted for triclabendazole tested in a battery of 6 genotoxicity in vitro and in vivo ...
-
14
CLINICAL STUDIES
An open label, randomized trial, conducted in Vietnam compared the efficacy of triclabendazole (two 10 mg/kg doses given 12 hours apart with food) to oral artesunate (4 mg/kg, given once daily for ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied - EGATEN (triclabendazole) tablets are supplied as pale red, speckled, capsule shaped, biconvex tablets with “EG ⅁Ǝ” debossed on one side and functionally scored on both sides. Each ...
-
17
PATIENT COUNSELING INFORMATION
Important Administration Instructions - Advise patients that EGATEN should be taken orally with food. The tablets can be swallowed whole or divided in half and taken with water or crushed and ...
-
PRINCIPAL DISPLAY PANELEgaten® (triclabendazole) Tablets - 250 mg per tablet - NDC 0078-0937-91 - Rx only - 4 scored tablets - Novartis
-
INGREDIENTS AND APPEARANCEProduct Information