edex®
(alprostadil for injection)
For Intracavernous Use Only
-
Sterile Powder and Diluent (sterile 0.9% sodium chloride) in Cartridges
-
PATIENT INFORMATION FOR edex® CARTRIDGES
-
Please ...
edex®
(alprostadil for injection)
For Intracavernous Use Only
Sterile Powder and Diluent (sterile 0.9% sodium chloride) in Cartridges
PATIENT INFORMATION FOR edex® CARTRIDGES
Please read carefully before using.
Rx Only
Please read carefully before using.
edex® can only be obtained with a prescription from your doctor. You or your partner should be fully trained on the proper injection technique before using edex® at home. Be sure to use only the dose prescribed by your doctor.
This leaflet provides a summary of information about your medicine. Please read this information carefully before you prepare the edex® solution. The reusable edex® injection device is used to prepare and administer the edex® solution. A convenient carrying case is provided for the reusable edex® injection device.
Carefully follow the instructions for administration which are described below. For further information or advice, ask your doctor or pharmacist.
Please keep this information in case you need to refer to it again.
Erectile Dysfunction: Causes and Treatments
There are several causes of erectile dysfunction, commonly known as impotence. These include impaired blood circulation in the penis, nerve damage, hormonal imbalances, excessive alcohol use, emotional problems, and certain medications that you may be taking for other conditions. Smoking has an adverse effect on erectile function by accentuating the effects of other risk factors such as blood vessel disease or high blood pressure. Erectile dysfunction is often due to more than one of these causes.
Treatment for erectile dysfunction includes penile injections, medical devices that produce an erection, surgical procedures (e.g. penile bypass or implants), hormone treatment, psychological counseling, lifestyle changes, or a change in medication. You should not stop taking any prescription medications, unless told to do so by your doctor.
Your doctor has prescribed edex®, a penile injection, to treat your erectile dysfunction.
Use of edex®
edex® is injected into a specific area of the penis (see injection directions below) and should produce an erection in 5 to 20 minutes. The erection can be expected to last up to one hour. You should not use edex® more than 3 times a week. Injections should be administered at least 24 hours apart.
Ideally, the injection should be administered just prior to foreplay. If your partner experiences insufficient vaginal lubrication or painful vaginal sensations during intercourse, the use of a lubricant may be helpful.
Who should NOT use edex®?
Men who have conditions that might result in long-lasting erections should not use edex®. Some of these conditions include sickle cell anemia or trait, leukemia, and tumor of the bone marrow (multiple myeloma). If you have any of these conditions, consult your doctor.
Men with penile implants, severe penile curvature, or those who have been advised not to engage in sexual activity should not use edex®.
edex® should not be used by women or children.
What are the risks of using edex®?
Erections that last more than 6 hours can cause serious damage to the penile tissue and may result in permanent impotence. Call the prescribing physician or, if unavailable, seek professional help immediately if you still have an erection 6 hours after injection. Various treatment options for reversing a prolonged erection are available.
A common side effect of edex® is mild to moderate pain during injection. The erection may also be associated with a painful sensation. If you experience severe pain, contact the prescribing physician.
Call your doctor if you notice any redness, lumps, swelling, tenderness or curvature of the erect penis.
A small amount of bleeding at the injection site may occur. To prevent bruising, apply firm pressure to the injection site for 5 minutes. Tell your doctor if you have a condition or are taking a medicine that interferes with blood clotting.
There is a possibility of needle breakage with use of edex®. To best avoid breaking the needle, you should pay careful attention to your doctor's instructions and try to handle the injection device properly. If the needle breaks during injection and you are able to see and grasp the broken end, you should remove it and contact your doctor. If you cannot see or cannot grasp the broken end, you should promptly contact your doctor.
NOTE: edex® offers no protection from the transmission of sexually transmitted diseases such as HIV (the virus that causes AIDS). Small amounts of bleeding at the injection site can increase the risk of transmission of blood-borne diseases between partners.
There is no approved injectable treatment using multiple medications. In addition, there are no data on the efficacy and safety of these combinations.
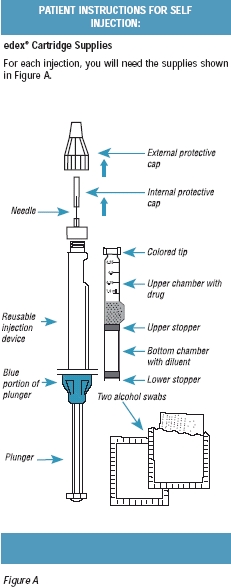
edex® Cartridge 2 Pack contains enough supplies for two injections. The 2 Pack contains the following items:
One reusable edex® injection device
One reusable carrying case
Two single-dose, dual-chamber cartridges (one per injection)
Two ½-inch, 29-gauge (0.33 mm x 12.7 mm) sterile needles (one per injection)
Four alcohol swabs (two per injection)
Patient Information for edex® Cartridges
edex® Cartridge 6 Pack contains enough supplies for six injections. The 6 Pack contains the following items:
One reusable edex® injection device
One reusable carrying case
Six single-dose, dual-chamber cartridges (one per injection)
Six ½-inch, 29-gauge (0.33 mm x 12.7 mm) sterile needles (one per injection)
Twelve alcohol swabs (two per injection)
Patient Information for edex® Cartridges
Storage and Handling
1. Store at 25°C (77°F); temperature variations between 15°C - 30°C (59°F - 86°F) are allowed. As with any drug product, extremes in temperature should be avoided. When traveling, do not store in checked luggage during air travel or leave in a closed automobile.
2. edex® solution should be used immediately after reconstitution.
IMPORTANT: To maintain sterility and avoid contamination, follow these directions carefully. Each needle and cartridge should be used only once. Safely discard the supplies (see the “Discard Injection Supplies” section of these instructions). The edex® cartridges contain a solid layer or cake of dry white powder approximately 3/8” in thickness. A normal cake may appear cracked or crumbled. If the cartridge is damaged, the cake may shrink in size. Do not use the cartridge if it appears damaged or the cake is substantially reduced in size.
Self-Injection Procedure
Before using edex®, you should be properly trained by your doctor. Mix edex® just prior to injection. Your dose has been customized for your individual needs. Use only the dose prescribed by your doctor. Have a clean area available to assemble the items necessary for your edex® injection. The reusable edex® injection device is for use only with the single-dose, dual-chamber cartridges and needles included in the edex® Cartridge 2 Pack or 6 Pack.
READ THE INSTRUCTIONS COMPLETELY BEFORE STARTING YOUR SELF-INJECTION PROCEDURE
Prepare edex® Solution
1. Wash your hands thoroughly with soap and water and dry them with a clean towel.
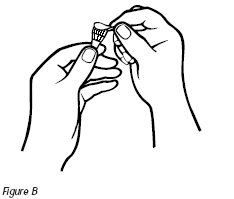
2. Check to see if the seal on the base of the needle is intact. Remove the seal from the base of the needle. Do not touch the exposed needle (Figure B).
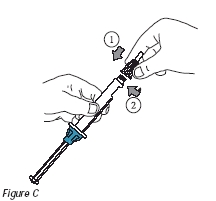
3. Attach the needle to the tip of the edex® injection device by turning clockwise until tight (Figure C). Note: Always attach the needle to the injection device before inserting the cartridge into the injection device.
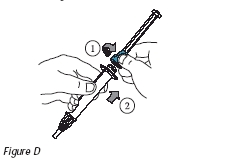
4. Turn the blue portion of the plunger counterclockwise to unscrew it from the injection device (Figure D).
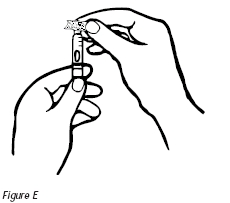
5. Pick up the cartridge and wipe the tip of the cartridge with an alcohol swab. Do not touch the tip of the cartridge after it has been cleansed with the alcohol swab (Figure E).
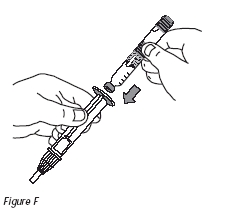
6. Insert the cartridge into the injection device with the tip facing toward the attached needle (Figure F). The ridge on the cartridge will need to fit into the groove on the injection device.
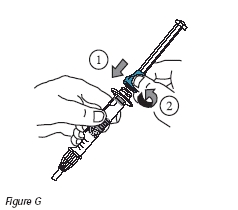
7. Attach the plunger to the injection device by turning the blue portion of the plunger clockwise until tight (Figure G).
8. Hold the injection device in an upright position with the needle pointing up.
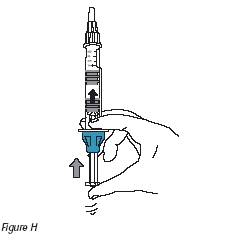
9. To prepare the drug solution, slowly push the plunger until the two gray rubber stoppers touch (Figure H). Gently move the injection device in a back and forth motion until the drug has dissolved and the solution is clear. The solution may initially appear cloudy due to small air bubbles. Do not use the solution if it remains cloudy, is colored, or contains particles.
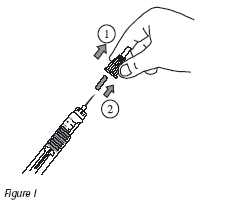
10. While holding the injection device with the needle pointing up, carefully remove the external and internal protective caps from the needle by pulling them straight off (Figure I). Do not turn the protective caps counterclockwise as this will loosen the needle. Do not discard the large external protective cap; you will need to use it later. Do not touch the exposed needle or allow the needle to touch anything.
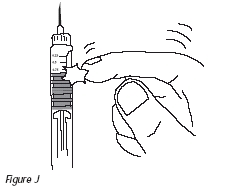
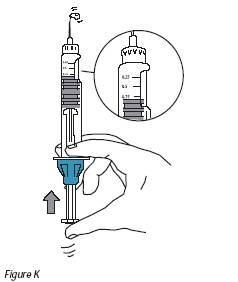
11. Gently tap the cartridge so that air bubbles float to the top of the solution (Figure J). Carefully push the plunger until a drop of solution appears at the end of the needle (Figure K).
Note: The plunger pushes the rubber stoppers forward; the rubber stoppers cannot be pulled back with the plunger.
12. Push the plunger until the upper rim of the top stopper reaches the correct volume mark for your prescribed dose. Excess solution will be expelled through the needle.
13. Set the injection device down on a clean, level surface and do not allow the needle to touch anything.
Select Injection Site
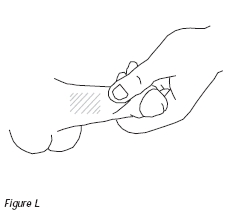
14. Choose an injection site mid-shaft on one side of the penis. Avoid visible blood vessels. With each use of edex®, alternate the side of the penis and vary the site of the injection (Figure L). If your penis is not circumcised, pull the foreskin back. Grasping the head of the penis with your thumb and forefinger, stretch it lengthwise along your thigh so that you can clearly see the selected injection site. Wipe the injection site with a new alcohol swab. Do not discard this swab; you will need to use it later.
Inject edex®
15. Pick up the injection device and reposition the penis as in Step 14 to keep it from moving during the injection.
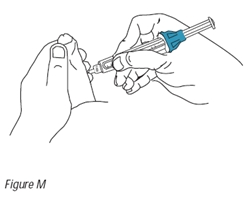
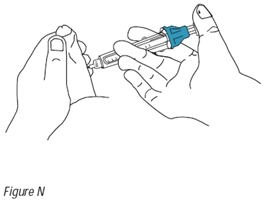
16. Hold the injection device as shown in Figure M. Do not touch the plunger at this time. Position the needle horizontally and gently insert the needle into the selected injection site until the needle is almost completely inserted into the penis (Figure M). Now place your thumb on the plunger and inject the solution slowly over a period of 5 to 10 seconds (Figure N).
17. If the solution does not inject easily, or if you immediately experience a burning pain at the injection site, reposition the needle by advancing it slightly or by partially withdrawing it until the solution can be injected easily and painlessly.
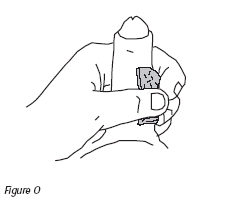
18. Withdraw needle from penis. Immediately apply firm but gentle pressure with the alcohol swab to the injection site for five minutes to prevent bruising (Figure O). Continue to apply firm pressure until bleeding stops. If bleeding continues or recurs after applying pressure, abstain from intercourse.
Discard Injection Supplies
19. Carefully place the large external protective cap on the needle. Remove the needle from the injection device by turning counterclockwise.
20. Remove the cartridge from the injection device by turning the blue portion of the plunger counterclockwise.
21. Discard your needle in a special container for disposal of sharp medical supplies. Ask your doctor or pharmacist where you can obtain these special containers. Follow the directions on your disposal container for proper disposal procedures. Do not reuse or share needles.
22. Clean the reusable injection device with warm water and a mild soap after each use. Once the injection device is dry, place it in the carrying case.
As with all prescription medicines, do not allow anyone else to use your medication. Proper injection technique and individual dose titration are essential for the safe use of this product.
Manufactured for:
Endo USA
Malvern, PA 19355
Revised: March 2024
Close