Label: ECOZA- econazole nitrate aerosol, foam
- NDC Code(s): 81811-100-70
- Packager: Resilia Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ECOZA safely and effectively. See full prescribing information for ECOZA. ECOZA - ®(econazole nitrate) topical foam, 1%, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEEcoza (econazole nitrate) topical foam, 1%, is indicated for the treatment of interdigital tinea pedis caused by - Trichophyton rubrum, Trichophyton mentagrophytes,and - Epidermophyton ...
-
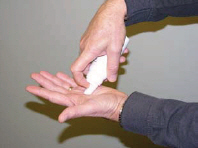
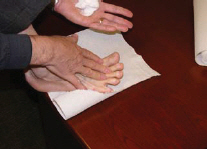
2 DOSAGE AND ADMINISTRATIONEcoza topical foam, 1% is for topical use only. Ecoza topical foam, 1% is not for oral, ophthalmic, or intravaginal use. Ecoza topical foam, 1% should be applied to cover affected areas once ...
-
3 DOSAGE FORMS AND STRENGTHSFoam, 1%. Each gram of Ecoza topical foam, 1%, contains 10 mg of econazole nitrate in a white to off-white foam.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Flammability - Ecoza topical foam is flammable. Avoid heat, flame, and smoking during and immediately following application. Contents under pressure. Do not puncture and/or incinerate the ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Warfarin - Concomitant administration of econazole and warfarin has resulted in enhancement of anticoagulant effect. Most cases reported product application with use under occlusion, genital ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C - There are no adequate and well-controlled trials with Ecoza topical foam in pregnant women. Ecoza topical foam should be used during pregnancy only if the ...
-
11 DESCRIPTIONEcoza (econazole nitrate) topical foam, 1% contains the azole antifungal agent, econazole nitrate in an oil-in-water emulsion base consisting of the following inactive ingredients: dimethicone ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ecoza topical foam is an azole antifungal [ see - Clinical Pharmacology (12.4)] . 12.2 Pharmacodynamics - The pharmacodynamics of Ecoza topical foam, 1% have ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies to determine the carcinogenic potential of Ecoza topical foam have not been performed. Oral administration of ...
-
14 CLINICAL STUDIESIn two multi-center, randomized, double-blind, vehicle-controlled clinical trials a total of 505 subjects with interdigital tinea pedis were randomized 1:1 to Ecoza topical foam or vehicle ...
-
16 HOW SUPPLIED/ STORAGE AND HANDLINGEcoza topical foam, 1% is white to off-white foam supplied in 10g (NDC 81811-100-10) and 70g (NDC 81811-100-70) aluminum pressurized canister. Store at controlled room temperature 20°C to 25°C ...
-
17 PATIENT COUNSELING INFORMATIONSee - FDA-approved patient labeling (Patient Information) The patient should be instructed as follows: Inform patients that Ecoza (econazole nitrate) topical foam, 1% is for topical use only ...
-
SPL UNCLASSIFIED SECTIONManufactured in the USA for - Resilia Pharmaceuticals, Inc. Atlanta, GA 30328 - PRODERM TECHNOLOGY - ® U.S, Patent 5,993,830 - ECOPI-022-2023-001 - Issued: 12/2023 - P/N 141333
-
PATIENT PACKAGE INSERTPatient Information - ECOZA - ® (ee-ko-zah) (econazole nitrate) topical foam, 1% Important information: Ecoza topical foam is for use on skin only. Do not use ...
-
Instructions for Use
ECOZA
®(ee-ko-zah)
(econazole nitrate) topical foam, 1%
Important information: Ecoza topical foam is for use on skin only. Do not use Ecoza topical foam in your eyes or vagina. Parts of Ecoza topical foam Canister. (See - Figure A ...
-
PRINCIPAL DISPLAY PANEL - 70 g Canister LabelNDC 81811-100-70 - ecoza™ (econazole nitrate) topical foam, 1% For Topical Use Only - Not for ophthalmic, oral or intravaginal use. Keep Out of Reach of Children - Rx Only - Net Wt 70g ...
-
PRINCIPAL DISPLAY PANEL - 70 g Canister CartonNDC 81811-0100-70 - ecoza™ (econazole nitrate) topical foam, 1% For Topical Use Only - Not for ophthalmic, oral or intravaginal use. Keep Out of Reach of Children - Rx Only - Net Wt 70g ...
-
INGREDIENTS AND APPEARANCEProduct Information