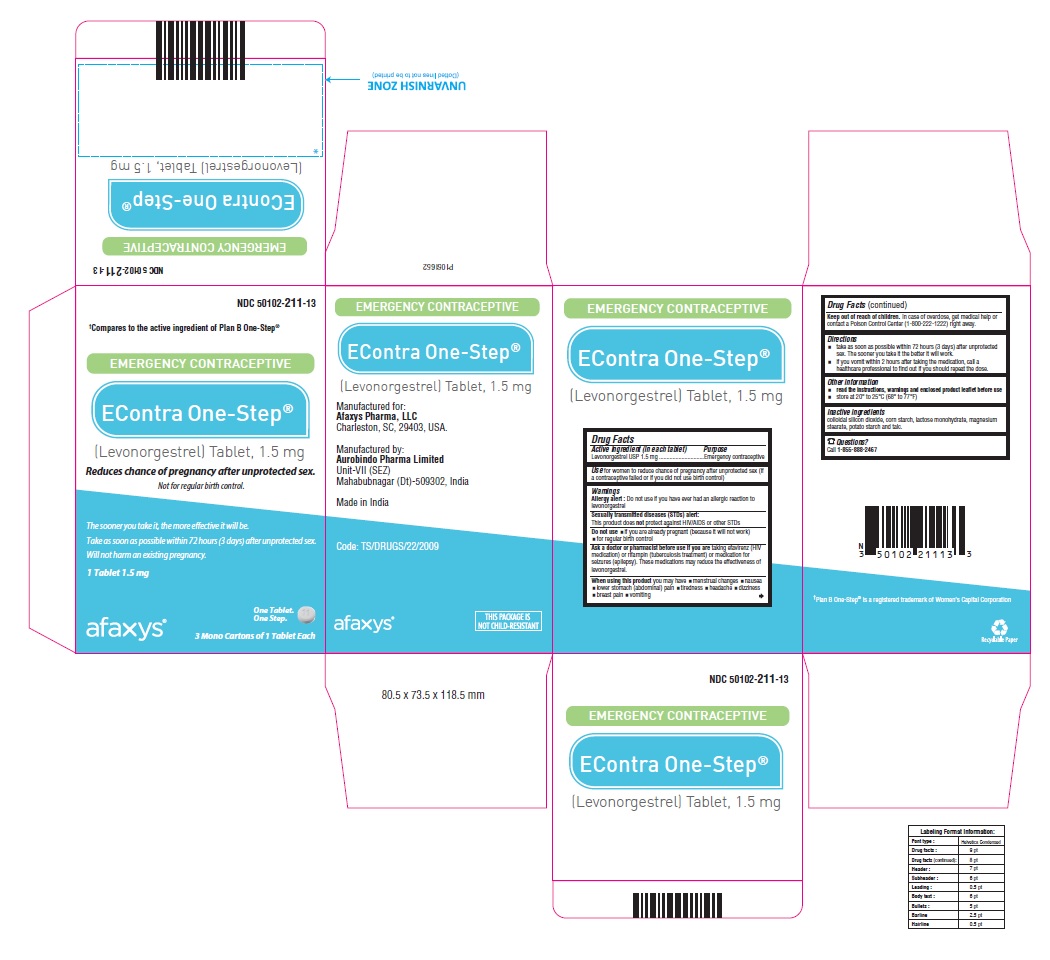
Label: ECONTRA ONE-STEP- levonorgestrel tablet
- NDC Code(s): 50102-211-01, 50102-211-11, 50102-211-13, 50102-211-16
- Packager: Afaxys Pharma, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 8, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredient (in each tablet) Levonorgestrel 1.5 mg
-
Purpose
Emergency contraceptive
-
Use
for women to reduce chance of pregnancy after unprotected sex (if a contraceptive failed or if you did not use birth control)
-
Warnings
Allergy alert: Do not use if you have ever had an allergic reaction to levonorgestrel - Sexually transmitted diseases (STDs) alert: This product does not protect against HIV/AIDS or other ...
-
Do not useif you are already pregnant (because it will not work) for regular birth control
-
Ask a doctor or pharmacist before use if you aretaking efavirenz (HIV medication) or rifampin (tuberculosis treatment) or medication for seizures (epilepsy). These medications may reduce the effectiveness of levonorgestrel.
-
WHEN USINGWhen using this product you may have - menstrual changes nausea - lower stomach (abdominal) pain - tiredness - headache - dizziness - breast pain - vomiting
-
Keep out of reach of children.In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
-
Directions
take as soon as possible within 72 hours (3 days) after unprotected sex. The sooner you take it the better it will work. if you vomit within 2 hours after taking the medication, call a healthcare ...
-
Other information
read the instructions, warnings and enclosed product leaflet before use - do not use if carton is open or seal is removed - store at 20° to 25°C (68° to 77°F)
-
Inactive ingredients
colloidal silicon dioxide, corn starch, lactose monohydrate, magnesium stearate, potato starch and talc.
-
Questions?
Call 1-855-888-2467 - Manufactured for: Afaxys Pharma, LLC - Charleston, SC, 29403, USA - Manufactured by: Aurobindo Pharma Limited - Unit-VII (SEZ) Mahabubnagar (Dt)-509302, India - Made in ...
-
Patient InformationEContra One-Step® (Levonorgestrel) Tablet, 1.5 mg - Emergency Contraceptive - One Tablet. One Step. What You Need to Know - What is EContra One-Step®? EContra One-Step®is emergency ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1.5 mg (3 Blister Carton Label)†Compares to the active ingredient of Plan B One-Step® NDC 50102-211-13 - EMERGENCY CONTRACEPTIVE - EContra One-Step® (Levonorgestrel) Tablet, 1.5 mg - Reduces chance of pregnancy after ...
-
INGREDIENTS AND APPEARANCEProduct Information