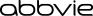Label: DURYSTA- bimatoprost implant
- NDC Code(s): 0023-9652-01, 0023-9652-03
- Packager: Allergan, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DURYSTA safely and effectively. See full prescribing information for DURYSTA. DURYSTA® (bimatoprost intracameral implant) ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
DURYSTA® (bimatoprost intracameral implant) is indicated for the reduction of intraocular pressure (IOP) in patients with open angle glaucoma (OAG) or ocular hypertension (OHT).
-
2
DOSAGE AND ADMINISTRATION
2.1 - General Information - DURYSTA is an ophthalmic drug delivery system for a single intracameral administration of a biodegradable implant. DURYSTA should not be readministered to an ...
-
3
DOSAGE FORMS AND STRENGTHS
Intracameral implant containing 10 mcg of bimatoprost in a drug delivery system.
-
4
CONTRAINDICATIONS
4.1 - Ocular or Periocular Infections - DURYSTA is contraindicated in patients with active or suspected ocular or periocular infections. 4.2 - Corneal Endothelial Cell ...
-
5
WARNINGS AND PRECAUTIONS
5.1 - Corneal Adverse Reactions - The presence of DURYSTA implants has been associated with corneal adverse reactions and increased risk of corneal endothelial cell loss. Administration ...
-
6
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in labeling: Implant migration [see Contraindications (4.4)] Hypersensitivity [see Contraindications ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no adequate and well-controlled studies of DURYSTA (bimatoprost intracameral implant) administration in pregnant women to inform a drug ...
-
11
DESCRIPTION
DURYSTA is a sterile intracameral implant containing 10 mcg of bimatoprost, a prostaglandin analog, in a solid polymer sustained-release drug delivery system (DDS). The drug delivery system ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Bimatoprost, a prostaglandin analog, is a synthetic structural analog of prostaglandin with ocular hypotensive activity. Bimatoprost is believed to lower IOP ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Bimatoprost was not carcinogenic in either mice or rats when administered by oral gavage at doses up to ...
-
14
CLINICAL STUDIES
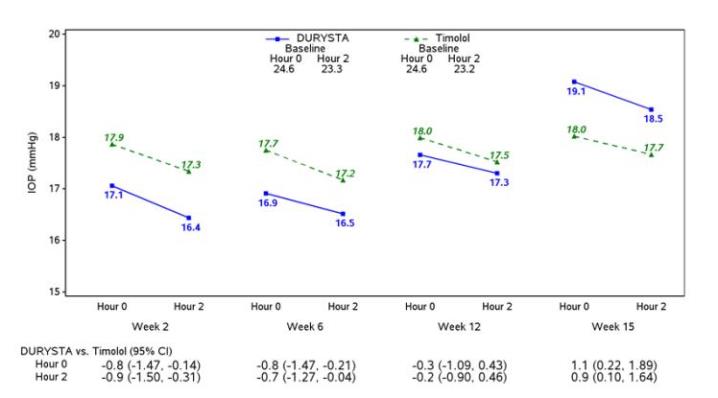
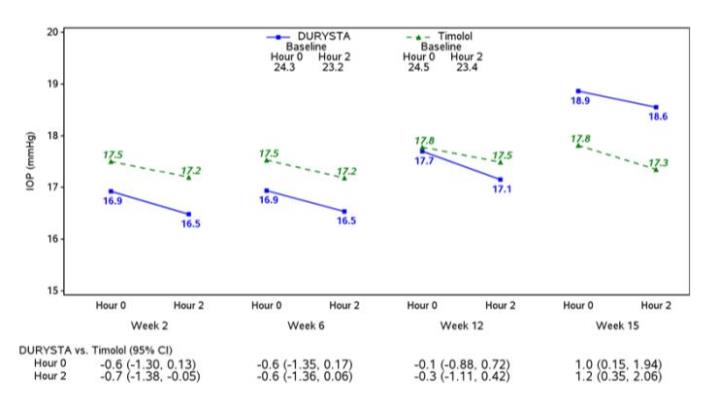
Efficacy was evaluated in two multicenter, randomized, parallel-group, controlled 20-month (including 8-month extended follow-up) studies of DURYSTA compared to twice daily topical timolol 0.5 ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
DURYSTA contains a 10 mcg bimatoprost intracameral implant in a single-use applicator that is packaged in a sealed foil pouch containing desiccant, NDC 0023-9652-01. Storage - Store refrigerated ...
-
17
PATIENT COUNSELING INFORMATION
Treatment-related Effects - Advise patients about the potential risk for complications including, but not limited to, the development of corneal adverse events, intraocular inflammation or ...
-
PRINCIPAL DISPLAY PANEL
NDC 0023-9652-01 - DURYSTA® (bimatoprost intracameral implant) 10 mcg - One Sterile, Single-Dose Applicator - For Intracameral administration - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information


![The chemical name for bimatoprost is (Z)-7-[(1R,2R,3R,5S)-3,5-dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 415.57. Its molecular formula is C25H37NO4.](/dailymed/image.cfm?name=durysta-03.jpg&setid=3f59da84-0bcc-4c84-b3e2-e215681ef341)