Label: DURACLON- clonidine hydrochloride injection, solution
- NDC Code(s): 67457-218-10
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- BOXED WARNING (What is this?)
-
BOXED WARNING
(What is this?)
NOTE: Duraclon® (epidural clonidine) is not recommended for obstetrical, post-partum, or peri-operative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients. However, in a rare obstetrical, post-partum or peri-operative patient, potential benefits may outweigh the possible risks.
Close -
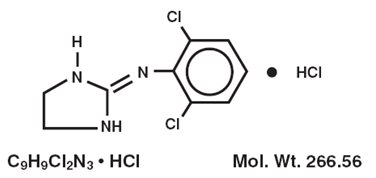
DESCRIPTIONDuraclon (clonidine hydrochloride injection, USP) is a centrally-acting analgesic solution for use in continuous epidural infusion devices. Clonidine Hydrochloride, USP, is an imidazoline ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Epidurally administered clonidine produces dose-dependent analgesia not antagonized by opiate antagonists. The analgesia is limited to the body regions innervated by the ...
-
INDICATIONS AND USAGEDuraclon is indicated in combination with opiates for the treatment of severe pain in cancer patients that is not adequately relieved by opioid analgesics alone. Epidural clonidine is more likely ...
-
CONTRAINDICATIONSDuraclon is contraindicated in patients with a history of sensitization or allergic reactions to clonidine. Epidural administration is contraindicated in the presence of an injection site ...
-
WARNINGSUse in Postoperative or Obstetrical Analgesia - Duraclon (epidural clonidine) is not recommended for obstetrical, post-partum, or peri-operative pain management. The risk of hemodynamic ...
-
PRECAUTIONSGeneral - Cardiac Effects: Epidural clonidine frequently causes decreases in heart rate. Symptomatic bradycardia can be treated with atropine. Rarely, atrioventricular block greater than first ...
-
ADVERSE REACTIONSAdverse reactions seen during continuous epidural clonidine infusion are dose-dependent and typical for a compound of this pharmacologic class. The adverse events most frequently reported in the ...
-
OVERDOSAGEHypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, irritability, and miosis. With large ...
-
DOSAGE AND ADMINISTRATIONThe recommended starting dose of Duraclon for continuous epidural infusion is 30 mcg/hr. Although dosage may be titrated up or down depending on pain relief and occurrence of adverse events ...
-
HOW SUPPLIEDNDC 67457-218-10, 100 mcg/mL solution in - 10 mL vials, packaged individually. Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Preservative Free. Discard unused ...
-
PRINCIPAL DISPLAY PANEL – 100 mcg/mL NDC 67457-218-10 10 mL - Duraclon® (clonidine HCl - injection, USP) 1000 mcg/10 mL - (100 mcg/mL) For Epidural Injection - Rx only Single-Dose Vial - Preservative Free - Sterile ...
-
INGREDIENTS AND APPEARANCEProduct Information