Label: DUODOTE- atropine and pralidoxime chloride kit
- NDC Code(s): 11704-620-01
- Packager: Meridian Medical Technologies® LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DUODOTE® safely and effectively. See full prescribing information for DUODOTE. DUODOTE (atropine and pralidoxime chloride ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
DuoDote is indicated for the treatment of poisoning by organophosphorus nerve agents as well as organophosphorus insecticides in adults and pediatric patients weighing more than 41 kg (90 ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information - Three (3) single-dose DuoDote autoinjectors should be available for use in each patient (including healthcare providers) at risk for organophosphorus ...
-
3 DOSAGE FORMS AND STRENGTHS
Each single-dose DuoDote autoinjector contains the following in two separate chambers: front chamber (visible): a clear, colorless to yellow, sterile solution of atropine (2.1 mg/0.7 mL) back ...
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Risks - Cardiovascular adverse reactions reported in the literature for atropine include, but are not limited to, sinus tachycardia, palpitations, premature ventricular ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling: Cardiovascular Risks [see Warnings and Precautions (5.1)] Heat Injury [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS
7.1 Succinylcholine and Mivacurium - Since pralidoxime in DuoDote reactivates cholinesterases and succinylcholine and mivacurium are metabolized by cholinesterases, patients with ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - Atropine readily crosses the placental barrier and enters fetal circulation. There are no adequate data on the developmental risk associated with the use of ...
-
10 OVERDOSAGE
10.1 Symptoms - Atropine - Manifestations of atropine overdose are dose-related and include flushing, dry skin and mucous membranes, tachycardia, widely dilated pupils that are poorly ...
-
11 DESCRIPTION
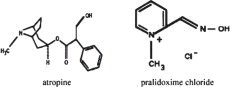
Each prefilled DuoDote autoinjector provides a single intramuscular dose of atropine, a cholinergic muscarinic antagonist, and pralidoxime chloride, a cholinesterase reactivator, in a ...
-
12 CLINICAL PHARMACOLOGY
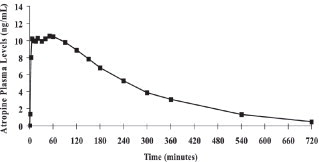
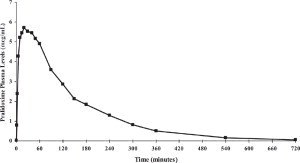
12.1 Mechanism of Action - Atropine - Atropine competitively blocks the effects of acetylcholine, including excess acetylcholine due to organophosphorus poisoning, at muscarinic cholinergic ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - DuoDote is indicated for short-term emergency use only, and no adequate studies regarding the carcinogenic ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each single-dose DuoDote (atropine and pralidoxime chloride) autoinjector contains atropine (2.1 mg/0.7 mL; colorless to yellow solution, visible in front chamber) and pralidoxime chloride (600 ...
-
17 PATIENT COUNSELING INFORMATION
Use by Healthcare Providers - DuoDote is intended for use by Healthcare Providers. See the illustrated Instruction Sheet for Healthcare Providers. Seek Definitive Medical Care - If feasible ...
-
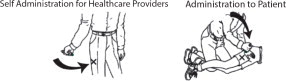
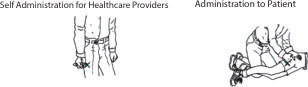
INSTRUCTIONS FOR USEInstruction Sheet for - Healthcare Providers - DuoDote is a single-dose autoinjector that should be administered by healthcare providers who have had adequate training in the recognition ...
-
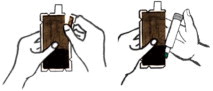
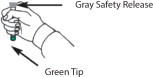
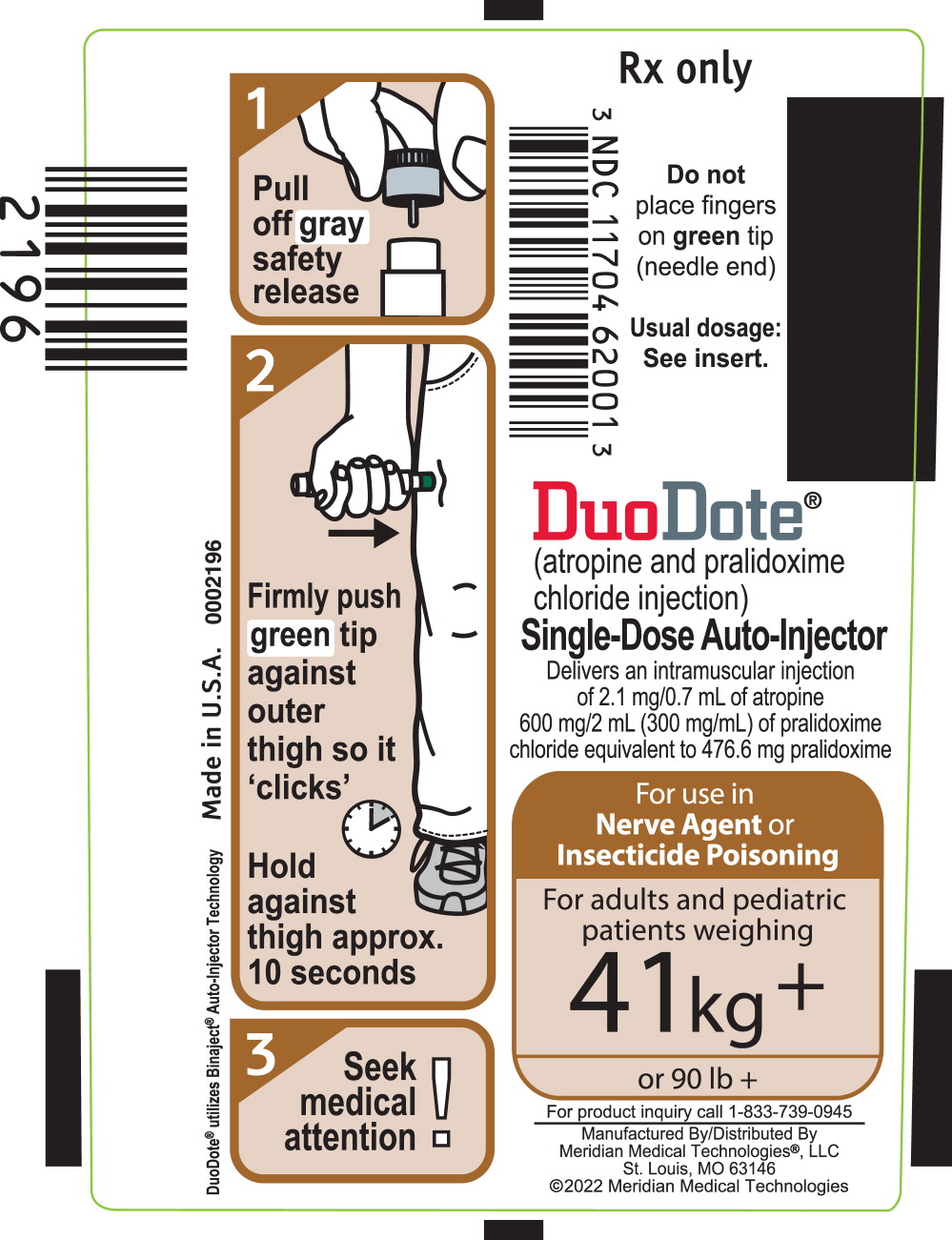
INSTRUCTIONS FOR USEInstructions for Use of the DuoDote Autoinjector - Do Not Remove Gray Safety Release until ready to use - Never touch the Green Tip (Needle End)! Weight Guidelines - For use with ...
-
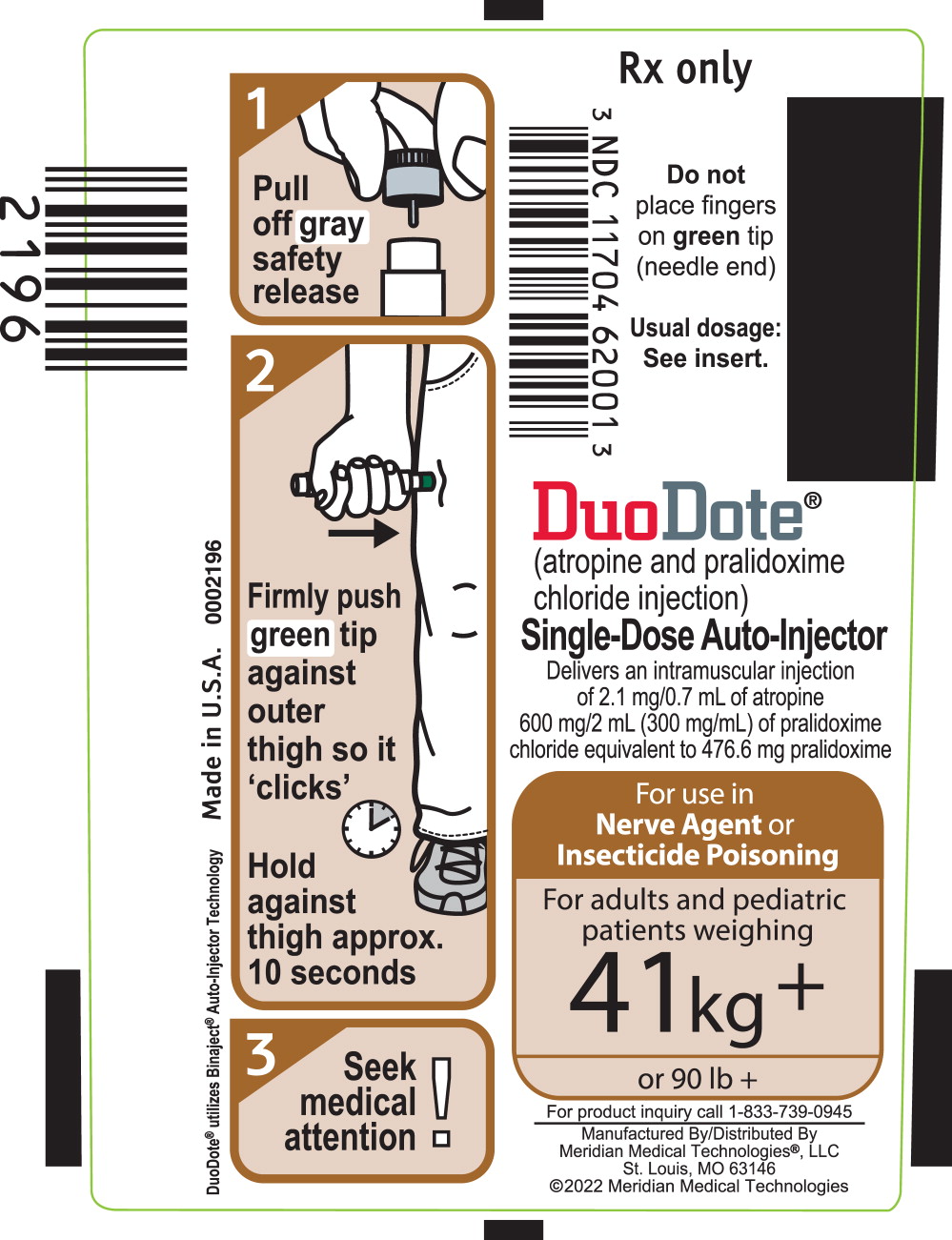
PRINCIPAL DISPLAY PANELPrincipal Display Panel - DuoDote Carton Label - For use in Nerve Agent - or Insecticide - Poisoning - For adults and pediatric - patients weighing - 41 kg + or 90 lb + NDC 11704-620-01 - Rx ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - DuoDote Pouch Label - For use in - Nerve Agent or - Insecticide Poisoning - For adults and pediatric - patients weighing - 41 kg + or 90 lb + Rx Only - MERIDIAN - MEDICAL ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - DuoDote Syringe Label - Rx Only - NDC 11704 62001 - Do not - place fingers - on green tip - (needled end) Usual dosage: See insert. DuoDote® (atropine and ...
-
INGREDIENTS AND APPEARANCEProduct Information