Label: DRISDOL- ergocalciferol capsule, liquid filled
- NDC Code(s): 30698-493-01
- Packager: Validus Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDRISDOL® (ergocalciferol, USP) 50,000 IU Capsule
-
DESCRIPTION
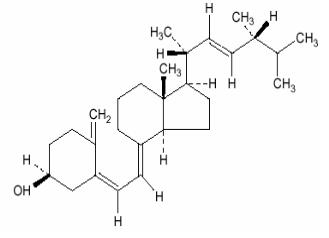
DRISDOL brand of ergocalciferol capsules, USP, is a synthetic calcium regulator for oral administration. Ergocalciferol is a white, colorless crystal, insoluble in water, soluble in organic ...
-
CLINICAL PHARMACOLOGY
The in vivo synthesis of the major biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of ergocalciferol takes place in the liver (to 25-hydroxyvitamin D ...
-
INDICATIONS AND USAGE
DRISDOL is indicated for use in the treatment of hypoparathyroidism, refractory rickets, also known as vitamin D resistant rickets, and familial hypophosphatemia.
-
CONTRAINDICATIONS
DRISDOL is contraindicated in patients with hypercalcemia, malabsorption syndrome, abnormal sensitivity to the toxic effects of vitamin D, and hypervitaminosis D.
-
WARNINGS
Hypersensitivity to vitamin D may be one etiologic factor in infants with idiopathic hypercalcemia. In these cases vitamin D must be strictly restricted. Keep out of the reach of children.
-
PRECAUTIONS
General - Vitamin D administration from fortified foods, dietary supplements, self-administered and prescription drug sources should be evaluated. Therapeutic dosage should be readjusted as ...
-
ADVERSE REACTIONS
Hypervitaminosis D is characterized by effects on the following organ system: Renal: Impairment of renal function with polyuria, nocturia, polydipsia, hypercalciuria, reversible azotemia ...
-
OVERDOSAGE
The effects of administered vitamin D can persist for two or more months after cessation of treatment. Hypervitaminosis D is characterized by: Hypercalcemia with anorexia, nausea, weakness ...
-
DOSAGE AND ADMINISTRATION
THE RANGE BETWEEN THERAPEUTIC AND TOXIC DOSES IS NARROW. Vitamin D Resistant Rickets: 12,000 to 500,000 IU units daily. Hypoparathyroidism: 50,000 to 200,000 IU units daily concomitantly with ...
-
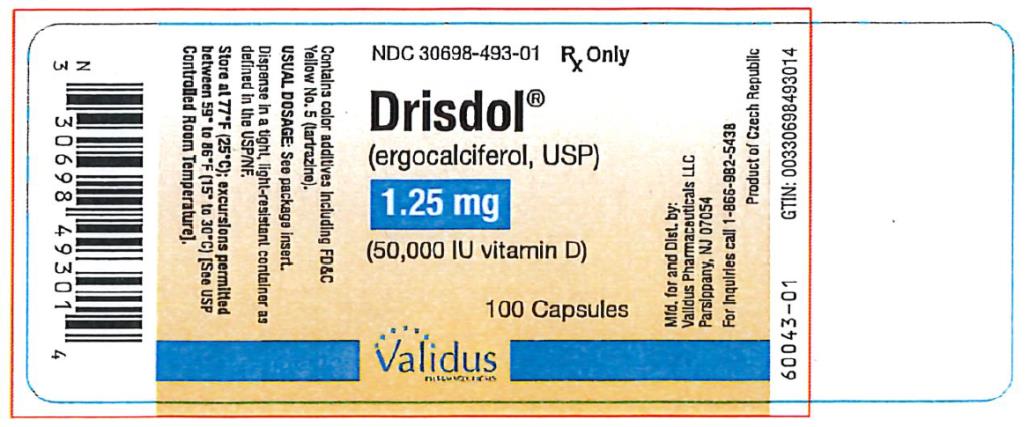
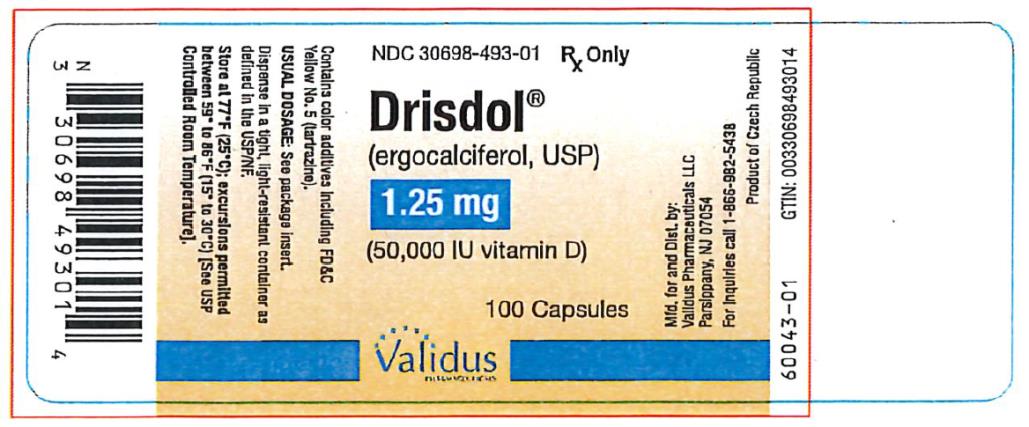
HOW SUPPLIED
Capsules of 1.25 mg (50,000 IU vitamin D) of ergocalciferol, USP are green and oval shaped, imprinted with "E 50" on one side and plain on the other. Supply in HDPE plastic bottles of 100 (NDC ...
-
PRINCIPAL DISPLAY PANEL
NDC 30698-493-01 - Drisdol - (ergocalciferol, USP) 1.25 - (50,000 IU vitamin D) 100 Capsules - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information