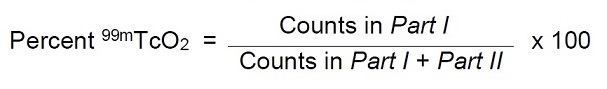Label: DRAXIMAGE DTPA- kit for the preparation of technetium tc 99m pentetate injection, powder, lyophilized, for solution
- NDC Code(s): 65174-288-30
- Packager: Jubilant DraxImage Inc., dba Jubilant Radiopharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information - needed to use DRAXIMAGE® DTPA safely and - effectively. See full prescribing information for DRAXIMAGE® DTPA. DRAXIMAGE® DTPA (kit for the ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDRAXIMAGE® DTPA, after radiolabeling with Technetium Tc 99m, is indicated for - 1.1 Brain - Imaging - Brain imaging in adults by intravenous administration. 1.2 Renal - Scintigraphy - Renal ...
-
2 DOSAGE AND ADMINISTRATION2.1 Radiation Safety - – Drug Handling - Tc 99m labeled DRAXIMAGE® DTPA injection is a radioactive drug and should be handled with appropriate safety measures to minimize radiation exposure to the ...
-
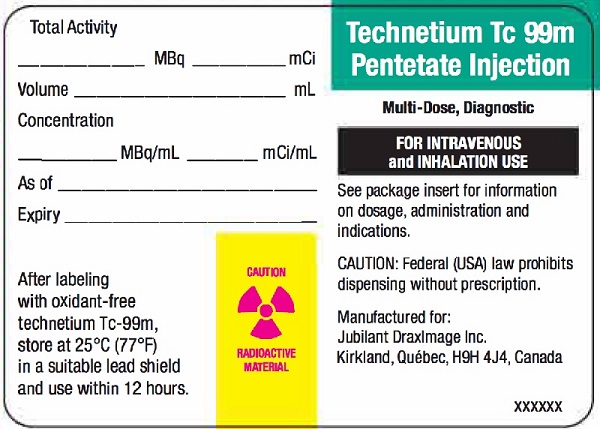
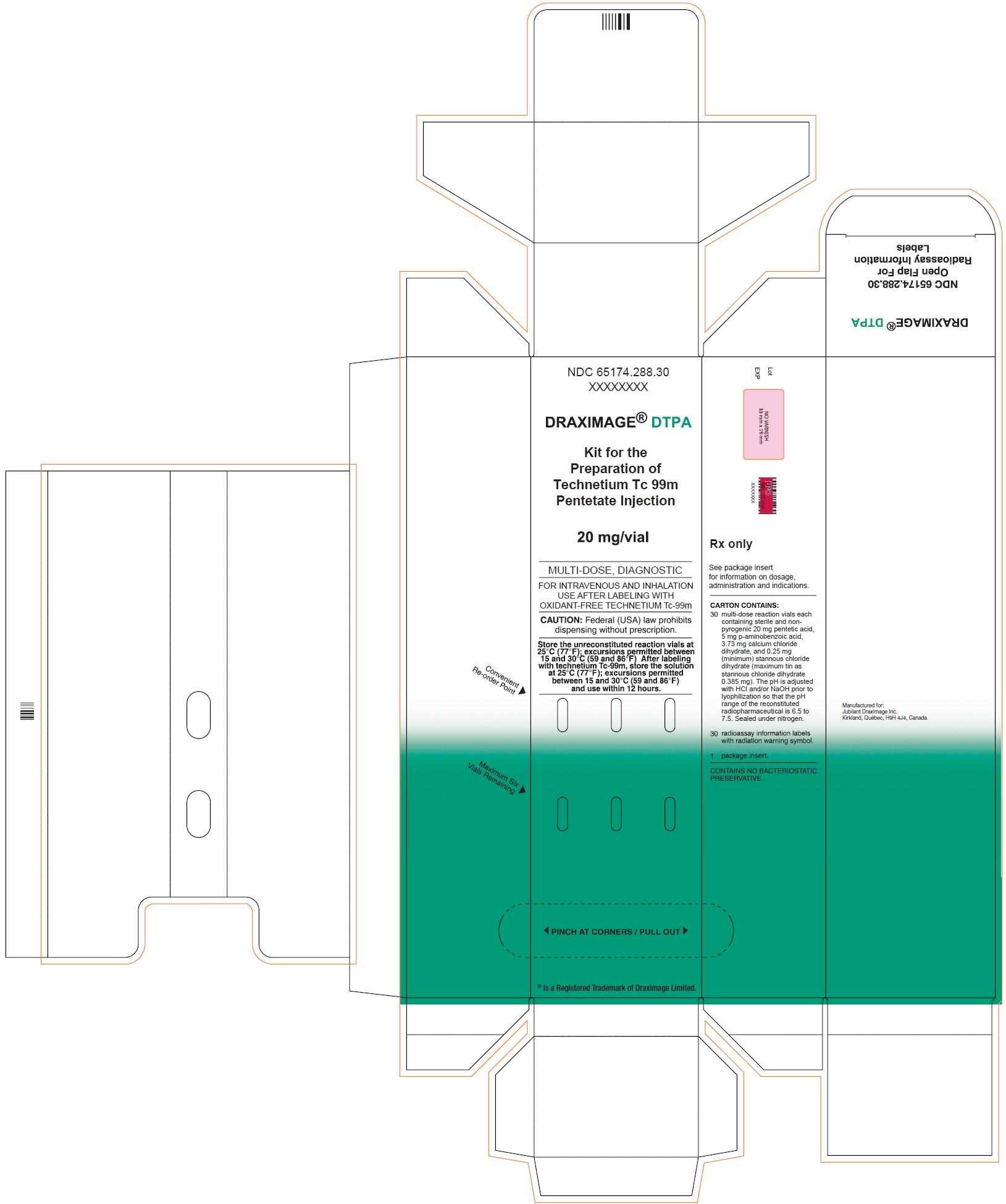
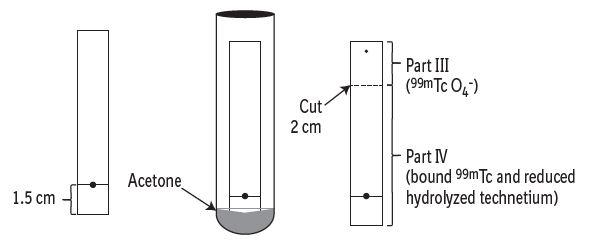
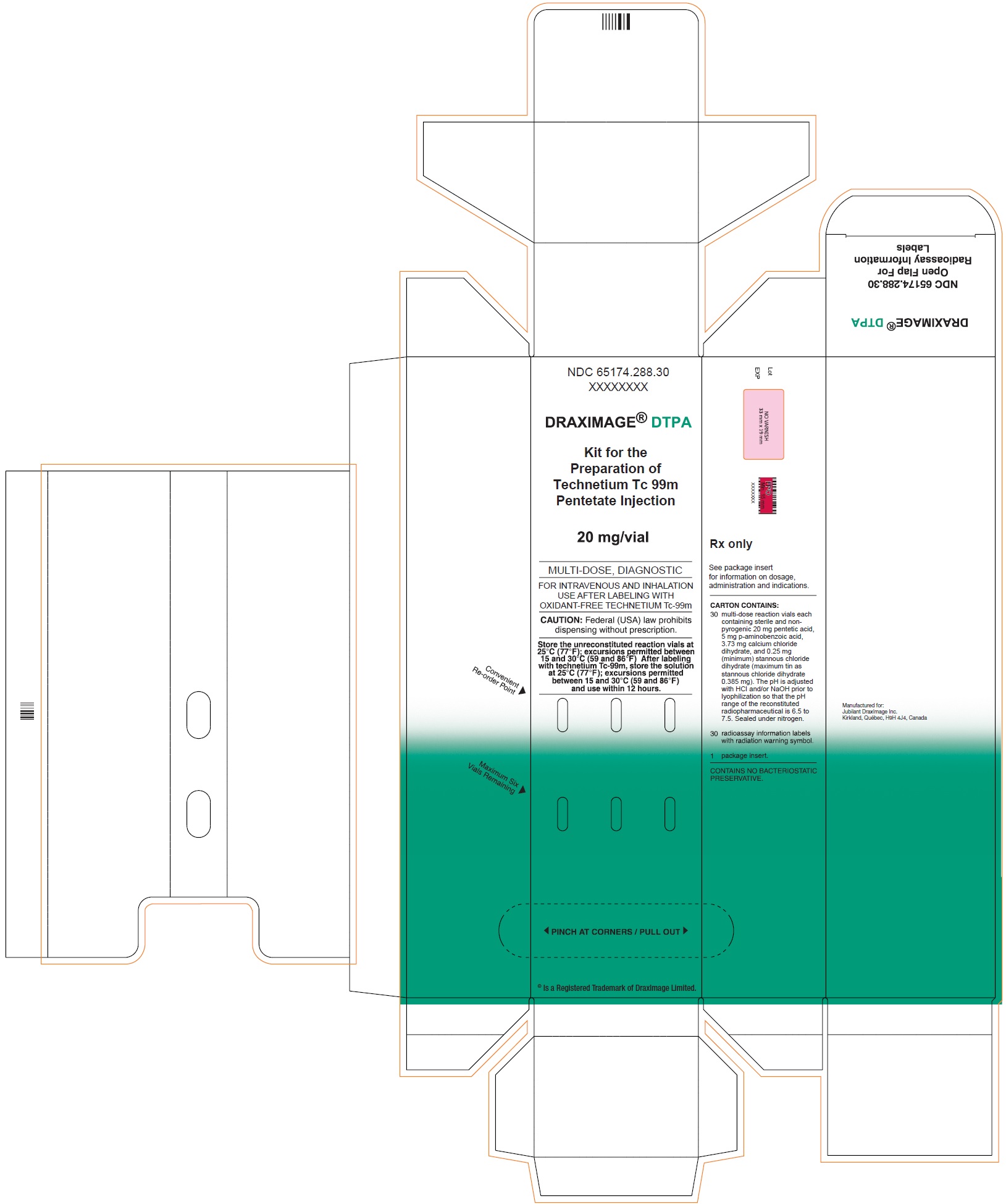
3 DOSAGE FORMS AND STRENGTHSKit for the preparation of Technetium Tc 99m pentetate injection: multiple-dose 10 mL glass vial contains a non-radioactive (white) lyophilized powder with 20 mg of pentetic acid, 5 mg of ...
-
4 CONTRAINDICATIONSHypersensitivity to the active - ingredient or to any component of the product [see Warnings - and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Reactions - Hypersensitivity reactions, including anaphylaxis, have been reported during post-approval diagnostic use of Technetium Tc 99m pentetate injection. Monitor all ...
-
6 ADVERSE REACTIONSThe following adverse reactions - have been identified post-approval. Because these reactions are voluntarily - reported from a population of uncertain size, it is not always possible - to reliably ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with Technetium Tc 99m pentetate use in pregnant women are insufficient to inform a drug associated risk for major birth defects and ...
-
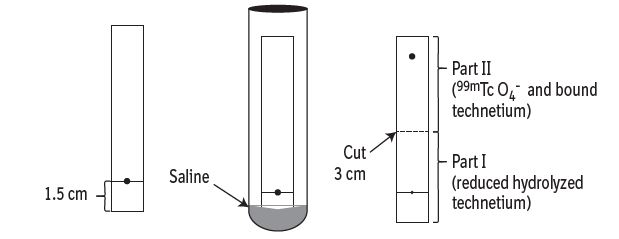
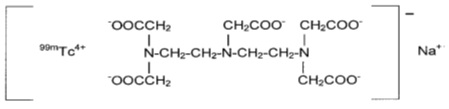
11 DESCRIPTION11.1 Chemical Characteristics - DRAXIMAGE® DTPA is a kit for the preparation of Technetium Tc 99m pentetate injection, a radioactive diagnostic agent, for intravenous or inhalation use. Each ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Intravenous Administration - Following intravenous administration for brain and renal imaging, Technetium Tc 99m pentetate is distributed in the vascular compartment ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - DRAXIMAGE® DTPA is supplied as multiple dose kits consisting of 10 mL reaction vials containing a white, lyophilized powder with 20 mg of pentetic acid, 5 mg of ...
-
17 PATIENT COUNSELING
INFORMATIONAdministration Instructions - Intravenous Use - Advise patients to hydrate after administration of Tc 99m labeled DRAXIMAGE® DTPA injection and to void frequently to minimize radiation dose [see ...
-
SPL UNCLASSIFIED SECTION® Registered Trademark of Jubilant DraxImage Inc. Manufactured for: Jubilant DraxImage Inc., Kirkland, Quebec, Canada, H9H 4J4. Revised: May 2023 - Art Rev.: 1.3
-
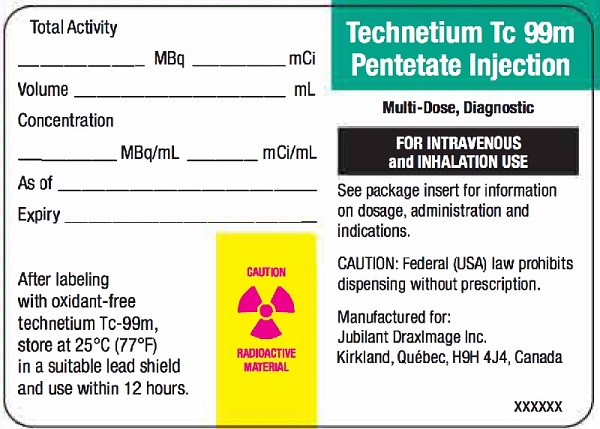
Radioactive Label
-
Vial Label

-
30 Vials Carton
-
INGREDIENTS AND APPEARANCEProduct Information