Label: DRAX EXAMETAZIME- kit for the preparation of technetium tc 99m exametazime for leukocyte labeling injection, powder, lyo...view full title
- NDC Code(s): 65174-200-01, 65174-200-05
- Packager: Jubilant DraxImage Inc., dba Jubilant Radiopharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Drax Exametazime safely and effectively. See full prescribing information for Drax Exametazime. Drax Exametazime (kit for the ...
-
Table of ContentsTable of Contents
-
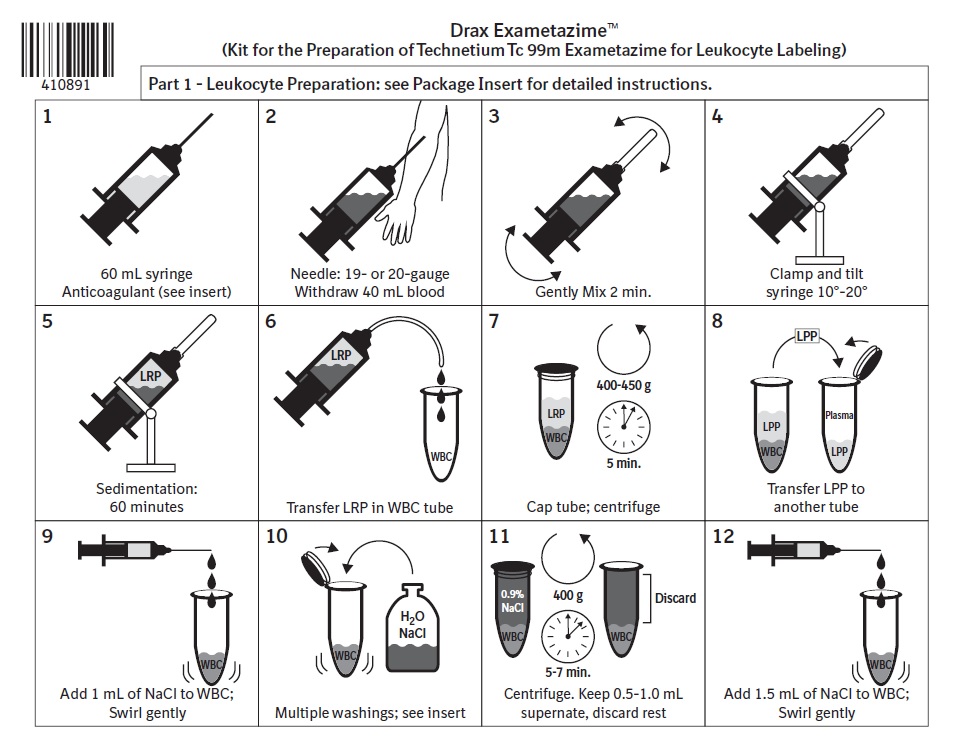
1 INDICATIONS AND USAGEDrax Exametazime is indicated for leukocyte (white blood cell) labeled scintigraphy as an adjunct in the localization of intra-abdominal infection and inflammatory bowel disease.
-
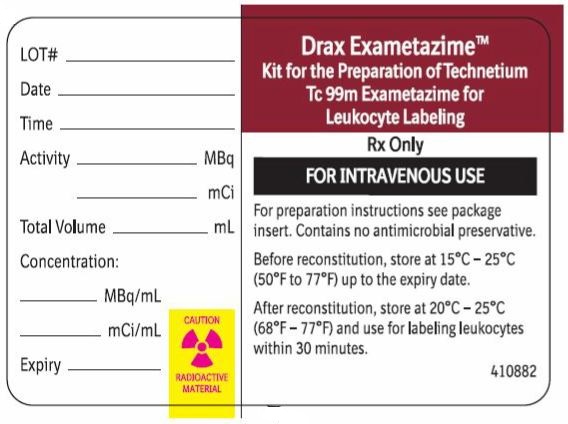
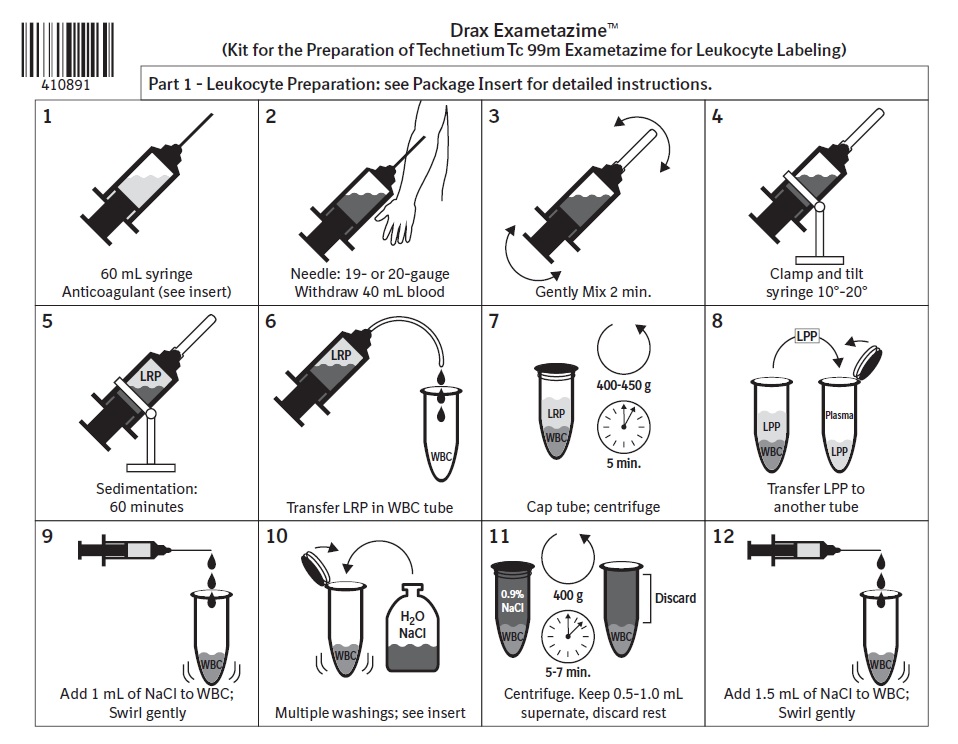
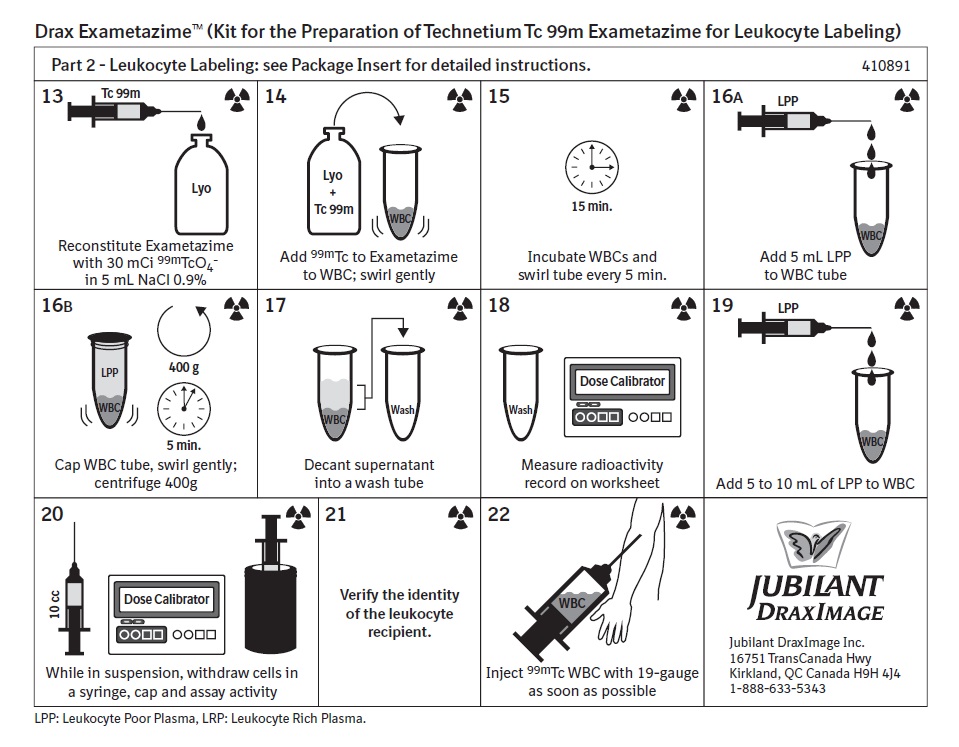
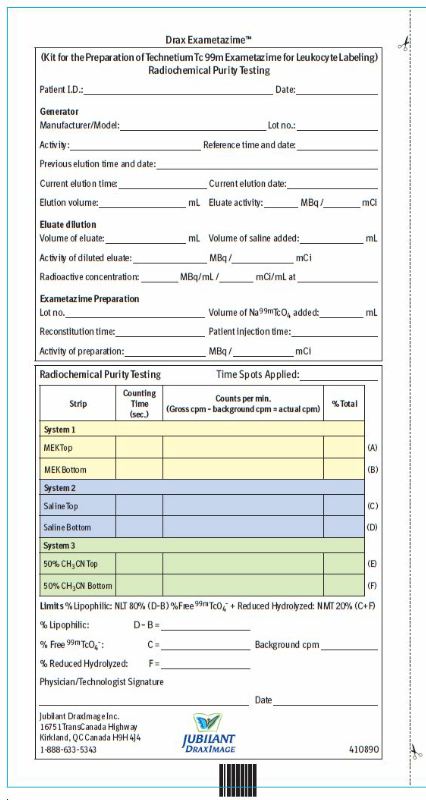
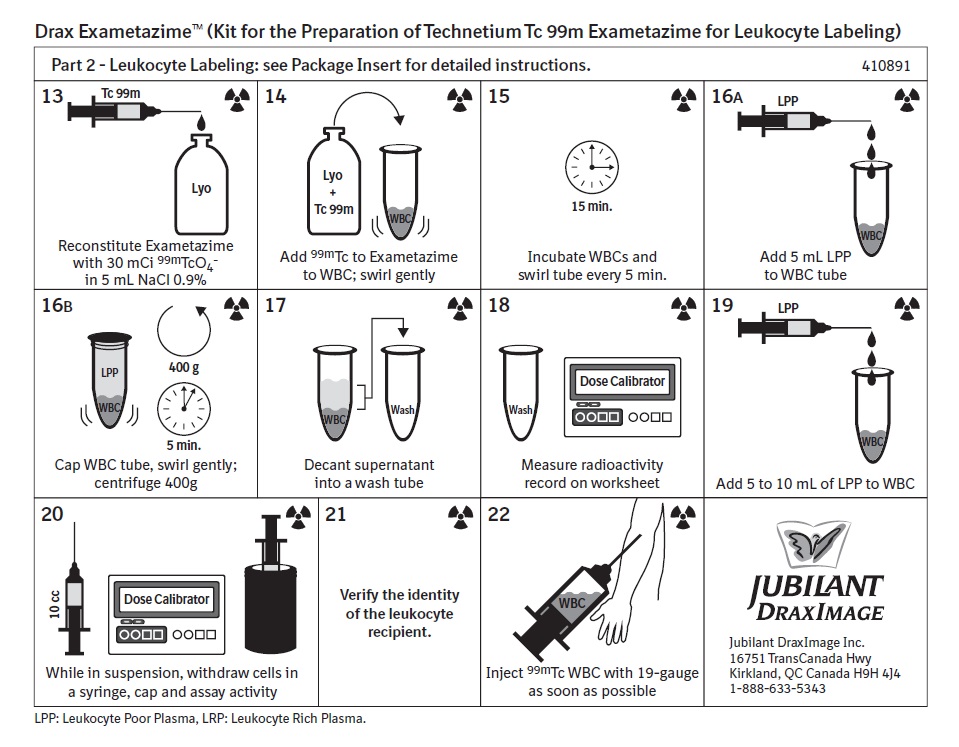
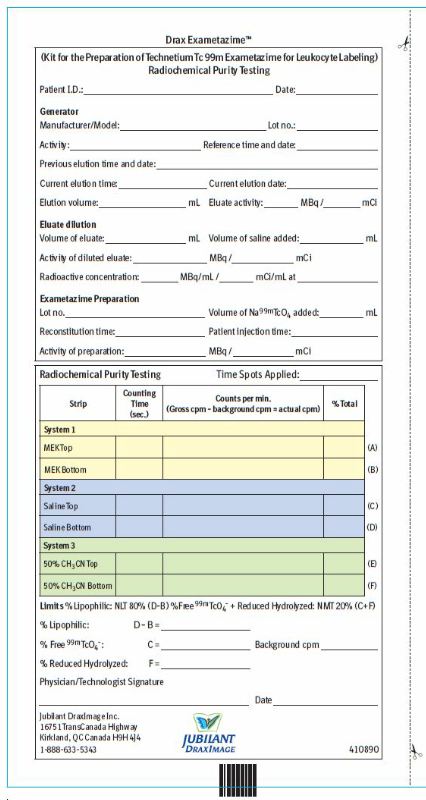
2 DOSAGE AND ADMINISTRATION2.1 Radiation Safety - Drug Handling - Technetium Tc 99m exametazime is a radioactive solution and should be handled with appropriate safety measures to minimize radiation exposure. During ...
-
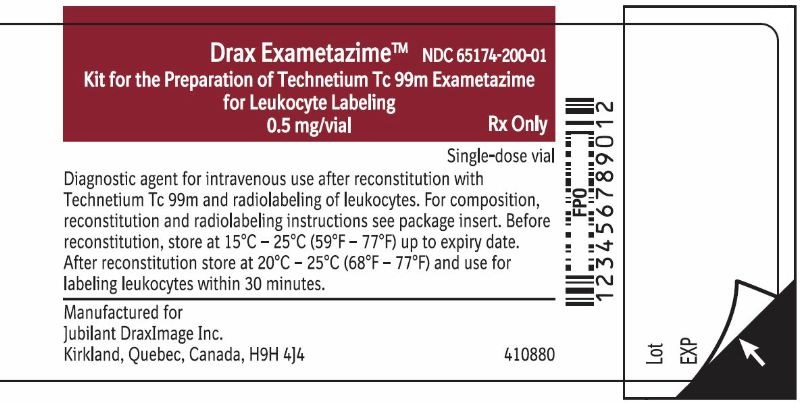
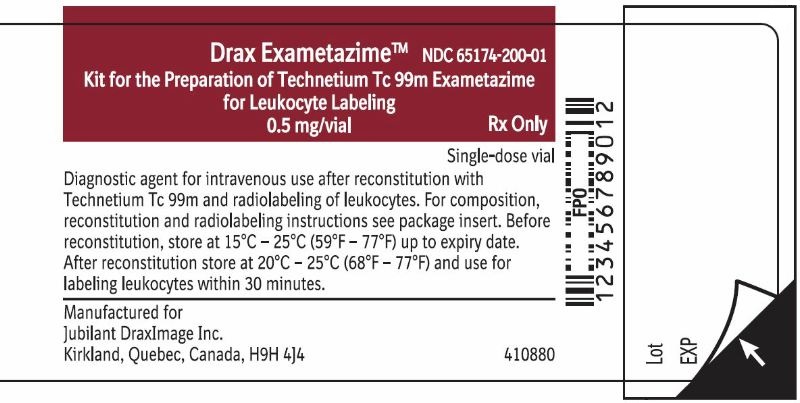
3 DOSAGE FORMS AND STRENGTHSDrax Exametazime is a kit containing five (5) single-dose vials. Each 10 mL, clear glass vial contains a non-radioactive lyophilized mixture of: 0.5 mg exametazime, 7.6 mcg stannous chloride ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including serious signs and symptoms of anaphylaxis, following administration of Tc 99m labeled leukocytes prepared using Tc 99m ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in the labeling; Hypersensitivity reactions [see Warnings and Precautions (5.1)]. The following adverse reactions associated with the use ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited available data with technetium Tc 99m exametazime use in pregnant women are insufficient to inform a drug associated risk for major birth defects and ...
-
10 OVERDOSAGEIn the event of the administration of a radiation overdose, hydration and frequent micturition should be encouraged in order to minimize the absorbed dose to patient.
-
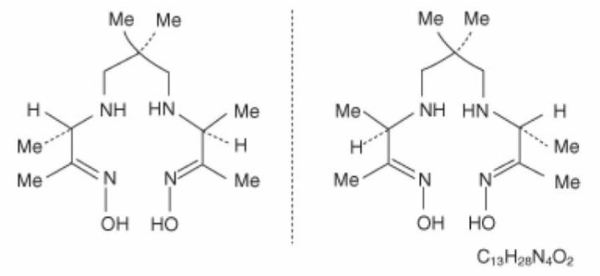
11 DESCRIPTION11.1 Chemical Characteristics - Drax Exametazime (kit for the preparation of technetium Tc 99m exametazime for leukocyte labeling) prepares a radioactive diagnostic agent. Each single-dose vial ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - When technetium Tc 99m pertechnetate is added to exametazime in the presence of stannous reductant, a lipophilic technetium Tc 99m complex is formed. This lipophilic ...
-
14 CLINICAL STUDIESTwo clinical trials of technetium Tc 99m exametazime were performed in a total of 88 patients who had suspected intra-abdominal infection or inflammation. Subjects received both Tc 99m labeled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Drax Exametazime kit (NDC 65174-200-05) comprises: 5 Single-dose vials (0.5 mg/vial). Each vial contains a non-radioactive sterile, non-pyrogenic lyophilized mixture of: 0.5 ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions: Advise patients to hydrate after administration of technetium Tc 99m exametazime labeled leukocytes and to void frequently to minimize radiation dose [see Dosage and ...
-
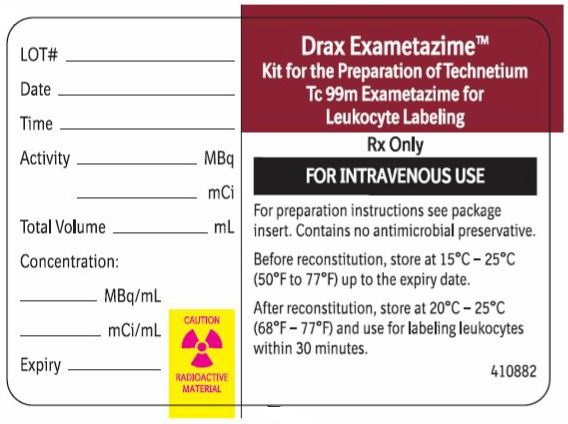
Carton LabelRx only - For Reconstitution and Radiolabelling Instructions:See package insert. Important: For intravenous use as directed. Use appropriate radiation shielding. Use only for radiolabeling of ...
-
INGREDIENTS AND APPEARANCEProduct Information