Label: DIFICID- fidaxomicin tablet, film coated
DIFICID- fidaxomicin granule, for suspension
- NDC Code(s): 52015-080-01, 52015-700-21, 52015-700-22, 52015-700-23
- Packager: Merck Sharp & Dohme LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DIFICID safely and effectively. See full prescribing information for DIFICID. DIFICID® (fidaxomicin) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Clostridioides difficile-Associated Diarrhea - DIFICID® is indicated in adult and pediatric patients aged 6 months and older for the treatment of C. difficile-associated diarrhea ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - DIFICID is available for oral administration as 200 mg tablets and as granules for oral suspension (40 mg/mL (200 mg/5 mL) when reconstituted). DIFICID ...
-
3 DOSAGE FORMS AND STRENGTHSDIFICID tablets - 200 mg white to off-white film-coated, oblong tablets; each tablet is debossed with "FDX" on one side and "200" on the other side. DIFICID for oral suspension - White to ...
-
4 CONTRAINDICATIONSDIFICID is contraindicated in patients who have known hypersensitivity to fidaxomicin or any other ingredient in DIFICID [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Acute hypersensitivity reactions, including dyspnea, rash, pruritus, and angioedema of the mouth, throat, and face have been reported with DIFICID. If a severe ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSFidaxomicin and its main metabolite, OP-1118, are substrates of the efflux transporter, P-glycoprotein (P-gp), which is expressed in the gastrointestinal tract. 7.1 Cyclosporine - Cyclosporine is ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data on use of DIFICID in pregnant women are insufficient to inform any drug-associated risk for major birth defects, miscarriage or ...
-
10 OVERDOSAGENo cases of acute overdose have been reported in humans. No drug-related adverse effects were seen in dogs dosed with fidaxomicin tablets at 9600 mg/day (over 100 times the human dose, scaled by ...
-
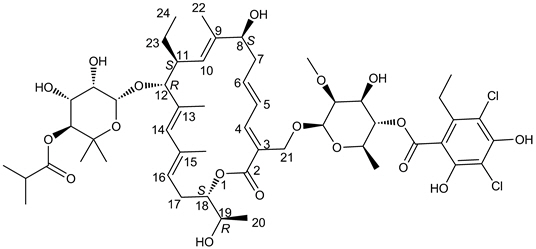
11 DESCRIPTIONDIFICID (fidaxomicin) is a macrolide antibacterial drug for oral administration. Its CAS chemical name is Oxacyclooctadeca-3,5,9,13,15-pentaen-2-one ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Fidaxomicin is an antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Fidaxomicin acts locally in the gastrointestinal tract on C. difficile. In a ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies have not been conducted to evaluate the carcinogenic potential of fidaxomicin. Neither fidaxomicin ...
-
14 CLINICAL STUDIES14.1 Clinical Studies of DIFICID in Adult Patients with CDAD - In two randomized, double-blinded trials, a non-inferiority design was utilized to demonstrate the efficacy of DIFICID (200 mg ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Tablets - DIFICID tablets are white to off-white film-coated, oblong tablets containing 200 mg of fidaxomicin per tablet; each tablet is debossed with "FDX" on one side and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Oral Suspension - Remove the bottle from the refrigerator 15 minutes prior to each administration. Instruct ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Merck Sharp & Dohme LLC - Rahway, NJ 07065, USA - For patent information: www.msd.com/research/patent - Copyright © 2015-2022 Merck & Co., Inc., Rahway, NJ, USA, and its ...
-
PATIENT PACKAGE INSERTPatient Information - DIFICID® (dih-fih-sid) (fidaxomicin) tablets, for oral use - DIFICID® (dih-fih-sid) (fidaxomicin) for oral suspension - What You Need to Know About Your ...
-
SPL UNCLASSIFIED SECTIONManuf. for: Merck Sharp & Dohme LLC - Rahway, NJ 07065, USA - For patent information: www.msd.com/research/patent. The trademarks depicted herein are owned by their respective ...
-
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle CartonNDC 52015-080-01 20 tablets - DIFICID® (fidaxomicin) tablets - 200 mg per tablet - Rx only
-
PRINCIPAL DISPLAY PANEL - 40 mg/mL Bottle Pouch CartonNDC 52015-700-22 - DIFICID® (fidaxomicin) for oral suspension - 40 mg/mL - MUST BE RECONSTITUTED BY A PHARMACIST - BEFORE DISPENSING - See enclosed package insert for preparation instructions. For ...
-
INGREDIENTS AND APPEARANCEProduct Information