Label: MOTOFEN- difenoxin and atropine sulfate tablet
- NDC Code(s): 54766-200-04, 54766-200-10
- Packager: Sebela Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: New Drug Application
Drug Label Information
Updated December 14, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
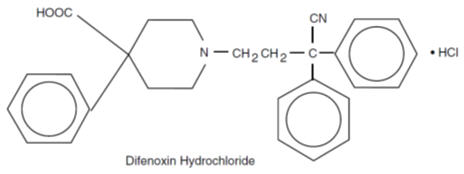
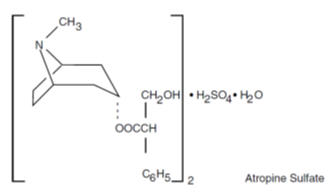
Each five-sided dye free MOTOFEN® tablet contains: 1 mg of difenoxin (equivalent to 1.09 mg of difenoxin hydrochloride) and 0.025 mg of atropine sulfate (equivalent to 0.01 mg of ...
-
CLINICAL PHARMACOLOGY
Animal studies have shown that difenoxin hydrochloride manifests its antidiarrheal effect by slowing intestinal motility. The mechanism of action is by a local effect on the gastrointestinal ...
-
INDICATIONS AND USAGE
MOTOFEN® is indicated as adjunctive therapy in the management of acute nonspecific diarrhea and acute exacerbations of chronic functional diarrhea.
-
CONTRAINDICATIONS
MOTOFEN® is contraindicated in patients with diarrhea associated with organisms that penetrate the intestinal mucosa (toxigenic - E. coli, Salmonella species, Shigella) and pseudomembranous ...
-
WARNINGS
MOTOFEN® IS NOT AN INNOCUOUS DRUG AND DOSAGE RECOMMENDATIONS SHOULD BE STRICTLY ADHERED TO. MOTOFEN® IS NOT RECOMMENDED FOR CHILDREN UNDER 2 YEARS OF AGE. OVERDOSAGE MAY RESULT IN SEVERE ...
-
PRECAUTIONS
CAUTION PATIENTS TO ADHERE STRICTLY TO RECOMMENDED DOSAGE SCHEDULES. THE MEDICATION SHOULD BE KEPT OUT OF REACH OF CHILDREN SINCE ACCIDENTAL OVERDOSAGE MAY RESULT IN SEVERE, EVEN FATAL ...
-
ADVERSE REACTIONS
In view of the small amount of atropine present (0.025 mg/tablet), such effects such as dryness of the skin and mucous membranes, flushing, hyperthermia, tachycardia and urinary retention are very ...
-
DRUG ABUSE AND DEPENDENCE
MOTOFEN® tablets are a Schedule IV controlled substance. Addiction to (dependence on) difenoxin hydrochloride is theoretically possible at high dosage. Therefore, the recommended dosage should ...
-
Diagnosis and Treatment
In the event of overdosage (initial signs may include dryness of the skin and mucous membranes, flushing, hyperthermia and tachycardia followed by lethargy or coma, hypotonic reflexes, nystagmus ...
-
DOSAGE AND ADMINISTRATION
The recommended starting dose of MOTOFEN® tablets in adults is 2 tablets then 1 tablet after each loose stool or 1 tablet every 3 to 4 hours as needed, but the total dosage during any 24-hour ...
-
HOW SUPPLIED
MOTOFEN® is available as a white, dye-free, five-sided, scored tablet with “0200” on the scored side and "M" on the other side. Each tablet contains 1 mg difenoxin and 0.025 mg atropine sulfate ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Sebela Pharmaceuticals Inc. 645 Hembree Parkway, Suite I - Roswell, GA 30076 - www.sebelapharma.com - Motofen is a registered trademark of Sebela International Limited - PI 20010 ...
-
54766-200-10 Bottle Label

-
54766-200-10 Carton

-
INGREDIENTS AND APPEARANCEProduct Information