Label: DIBENZYLINE- phenoxybenzamine hydrochloride capsule
- NDC Code(s): 59212-001-01, 59212-001-02
- Packager: Advanz Pharma (US) Corp.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
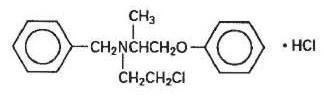
DESCRIPTIONEach Dibenzyline capsule, with red cap and body, is imprinted WPC 001 and 10 mg, and contains 10 mg of Phenoxybenzamine Hydrochloride USP. Inactive ingredients consist of D&C Red No. 33, and FD&C ...
-
CLINICAL PHARMACOLOGYDibenzyline (phenoxybenzamine hydrochloride) is a long-acting, adrenergic, alpha-receptor-blocking agent, which can produce and maintain "chemical sympathectomy" by oral administration. It ...
-
INDICATION AND USAGEDibenzyline is indicated in the treatment of pheochromocytoma, to control episodes of hypertension and sweating. If tachycardia is excessive, it may be necessary to use a beta-blocking agent ...
-
CONTRAINDICATIONSConditions where a fall in blood pressure may be undesirable; hypersensitivity to the drug or any of its components.
-
WARNINGDibenzyline-induced alpha-adrenergic blockade leaves beta-adrenergic receptors unopposed. Compounds that stimulate both types of receptors may, therefore, produce an exaggerated hypotensive ...
-
PRECAUTIONSGeneral - Administer with caution in patients with marked cerebral or coronary arteriosclerosis or renal damage. Adrenergic blocking effect may aggravate symptoms of respiratory ...
-
ADVERSE REACTIONSThe following adverse reactions have been observed, but there are insufficient data to support an estimate of their frequency. Autonomic Nervous System*: Postural hypotension, tachycardia ...
-
OVERDOSAGESYMPTOMS − These are largely the result of blocking of the sympathetic nervous system and of the circulating epinephrine. They may include postural hypotension, resulting in dizziness or fainting ...
-
TREATMENTWhen symptoms and signs of overdosage exist, discontinue the drug. Treatment of circulatory failure, if present, is a prime consideration. In cases of mild overdosage, recumbent position with legs ...
-
DOSAGE AND ADMINISTRATIONThe dosage should be adjusted to fit the needs of each patient. Small initial doses should be slowly increased until the desired effect is obtained or the side effects from blockade become ...
-
STORAGEStore at 25°C (77°F); excursions permitted to 15°- 30°C (59°- 86°F) [See USP Controlled Room Temperature]. Dispense in a tight container.
-
HOW SUPPLIEDDibenzyline (phenoxybenzamine hydrochloride) capsules, 10 mg, in bottles of 100 (NDC 59212-001-01) and (NDC 59212-001-02)
-
REFERENCES1. Weiner, N.: Drugs That Inhibit Adrenergic Nerves and Block Adrenergic Receptors, in Goodman, L., and Gilman, A., The Pharmacological Basis of Therapeutics, ed. 6, New York, Macmillan Publishing ...
-
SPL UNCLASSIFIED SECTION**Available as Levophed® (brand of norepinephrine bitartrate) from Hospira Inc. Made in Canada - Manufactured for: Advanz Pharma (US) Corp. Bannockburn, IL 60015 - DIBENZYLINE® is a registered ...
-
PACKAGE LABELNDC 59212-001-01 - Dibenzyline® (phenoxybenzamine hydrochloride capsules, USP) 10 mg - 100 capsules Rx only - Advanz Pharma (US) Corp.
-
INGREDIENTS AND APPEARANCEProduct Information