Label: DEXYCU- dexamethasone injection, suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 71879-001-01 - Packager: EyePoint Pharmaceuticals US, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DEXYCU ® safely and effectively. See full prescribing information for DEXYCU. DEXYCU (dexamethasone intraocular suspension) 9% ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDEXYCU (dexamethasone intraocular suspension) 9% is indicated for the treatment of postoperative inflammation.
-
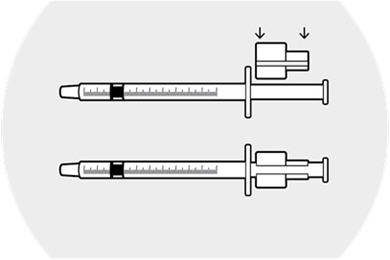
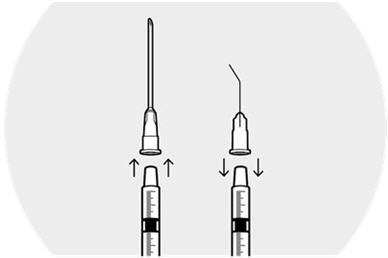
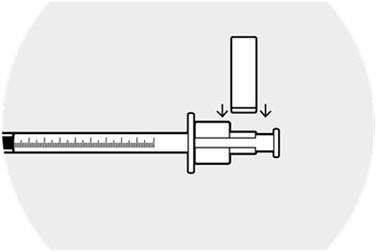
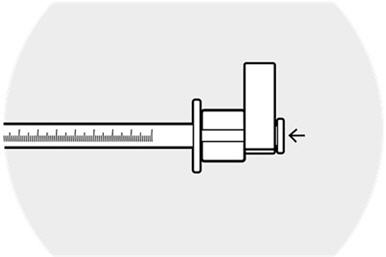
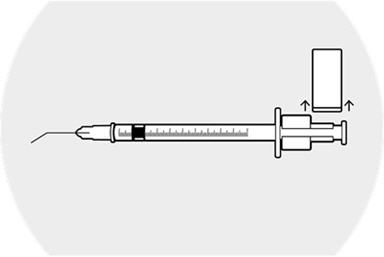
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - DEXYCU should be administered as a single dose, intraocularly in the posterior chamber at the end of surgery. The dose is 0.005 mL of dexamethasone 9% (equivalent to ...
-
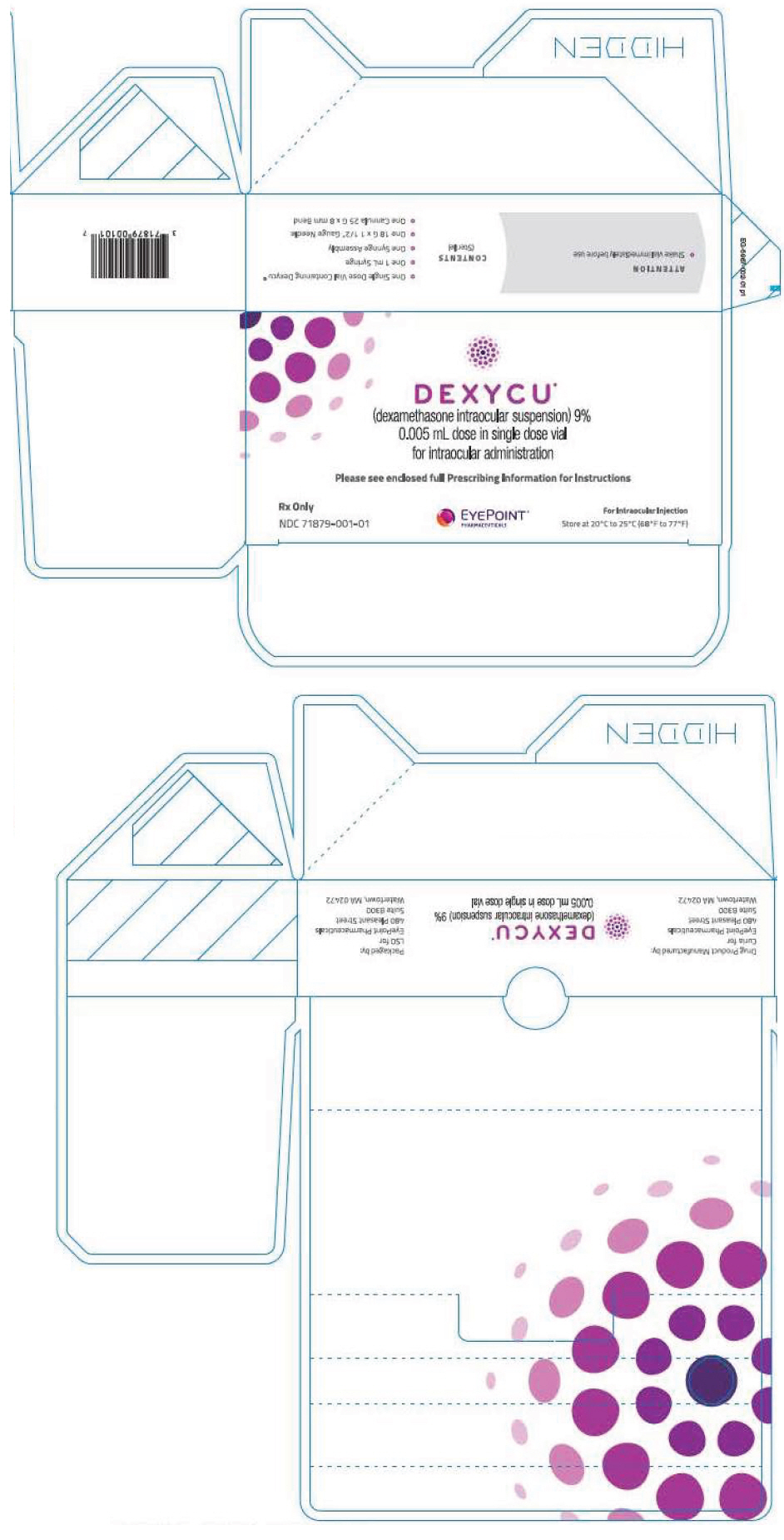
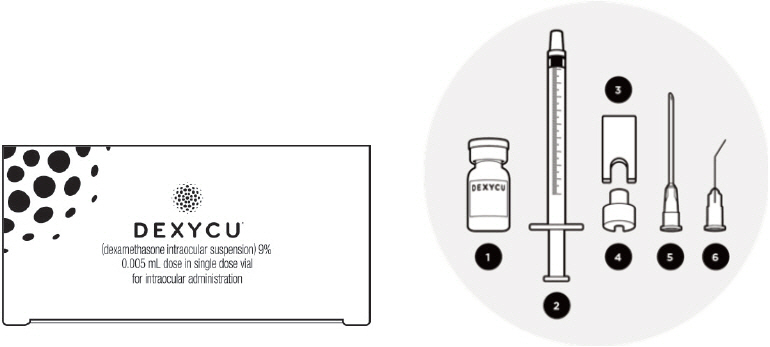
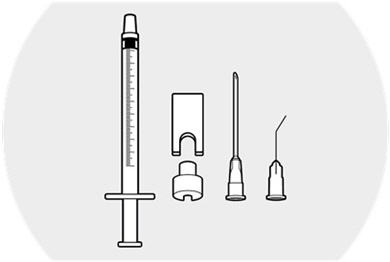
3 DOSAGE FORMS AND STRENGTHSDEXYCU contains dexamethasone 9% w/w (103.4 mg/mL) as a sterile suspension for intraocular ophthalmic administration. DEXYCU is provided as a kit for administration of a single dose of 0.005 mL of ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Increase in Intraocular Pressure - Prolonged use of corticosteroids including DEXYCU may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision ...
-
6 ADVERSE REACTIONSThe following adverse reactions are described elsewhere in the labeling: Increase in Intraocular Pressure [see Warnings and Precautions (5.1)] Delayed Healing [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of DEXYCU (dexamethasone intraocular suspension) 9% in pregnant women. Topical ocular administration of ...
-
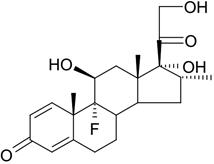
11 DESCRIPTIONDEXYCU (dexamethasone intraocular suspension) 9% is a corticosteroid, sterile, white to off-white opaque suspension for intraocular administration. Each vial of DEXYCU contains 0.5 mL of 9% w/w ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Dexamethasone is a corticosteroid. Corticosteroids have been shown to suppress inflammation by inhibiting multiple inflammatory cytokines resulting in decreased edema ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Animal studies have not been conducted to determine whether DEXYCU has the potential for carcinogenesis or mutagenesis. Fertility ...
-
14 CLINICAL STUDIESClinical efficacy was evaluated in a randomized, double-masked, placebo-controlled trial (NCT02006888) in which subjects received either DEXYCU or placebo (vehicle). A dose of 5 microliters of ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach kit of DEXYCU contains a single dose for a single patient. The 2-mL glass vial is filled with 0.5 mL of 9% dexamethasone intraocular suspension and has a blue cap (NDC ...
-
SPL UNCLASSIFIED SECTIONManufactured for: EyePoint Pharmaceuticals US, Inc. Watertown, MA 02472 - Patented. See https://eyepointpharma.com/patent-notification/
-
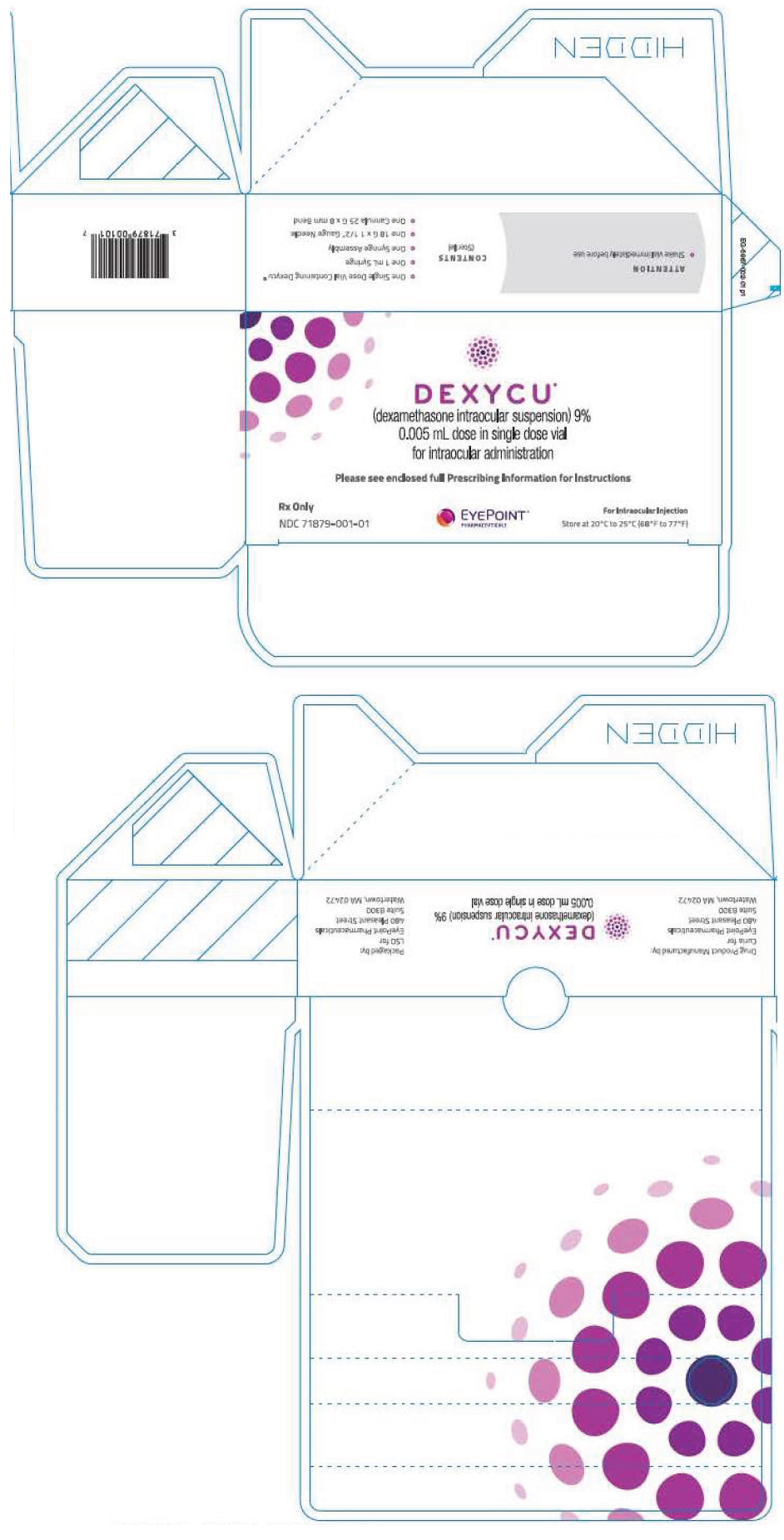
PRINCIPAL DISPLAY PANEL - 0.005 mL Vial CartonDEXYCU™ (dexamethasone intraocular suspension) 9% 0.005 mL dose in single dose vial - for intraocular administration - Please see enclosed full Prescribing Information for Instructions - Rx Only - NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information