Label: DEXEDRINE SPANSULE- dextroamphetamine sulfate capsule, extended release
- NDC Code(s): 64896-673-10, 64896-674-10, 64896-675-10
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated October 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: ABUSE, MISUSE, AND ADDICTION
DEXEDRINE has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including DEXEDRINE, can result in overdose and death [see Overdosage], and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing DEXEDRINE, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout DEXEDRINE treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction [see Warnings and Drug Abuse and Dependence]. Close -
DESCRIPTION
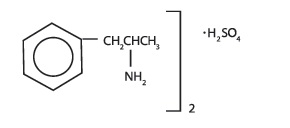
DEXEDRINE (dextroamphetamine sulfate) is the dextro isomer of the compound d,l-amphetamine sulfate, a sympathomimetic amine of the amphetamine group. Chemically, dextroamphetamine is ...
-
CLINICAL PHARMACOLOGY
Amphetamines are noncatecholamine, sympathomimetic amines with CNS stimulant activity. Peripheral actions include elevations of systolic and diastolic blood pressures and weak bronchodilator and ...
-
INDICATIONS AND USAGE
DEXEDRINE is indicated in: Narcolepsy - Attention Deficit Disorder with Hyperactivity - As an integral part of a total treatment program that typically includes other measures (psychological ...
-
CONTRAINDICATIONS
In patients known to be hypersensitive to amphetamine, or other components of DEXEDRINE. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients ...
-
WARNINGS
Abuse, Misuse, and Addiction - DEXEDRINE has a high potential for abuse and misuse. The use of DEXEDRINE exposes individuals to the risks of abuse and misuse, which can lead to the development of ...
-
PRECAUTIONS
General - The least amount feasible should be prescribed or dispensed at 1 time in order to minimize the possibility of overdosage. Information for Patients - Advise the patient to read the ...
-
ADVERSE REACTIONS
Cardiovascular - Palpitations, tachycardia, elevation of blood pressure. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use. Central Nervous ...
-
DRUG ABUSE AND DEPENDENCE
Controlled Substance - DEXEDRINE contains dextroamphetamine, a Schedule II controlled substance. Abuse - DEXEDRINE has a high potential for abuse and misuse which can lead to the development of a ...
-
OVERDOSAGE
Clinical Effects of Overdose - Overdose of CNS stimulants is characterized by the following sympathomimetic effects: Cardiovascular effects including tachyarrhythmias, and hypertension or ...
-
DOSAGE AND ADMINISTRATION
Amphetamines should be administered at the lowest effective dosage and dosage should be individually adjusted. Late evening doses should be avoided because of the resulting insomnia. Prior to ...
-
HOW SUPPLIED
DEXEDRINE SPANSULE capsules - Each capsule, with brown cap and natural body, contains dextroamphetamine sulfate. The 5-mg capsule is imprinted in white with IX and 5 mg on the brown cap and ...
-
MEDICATION GUIDE
MEDICATION GUIDE - DEXEDRINE®(dek-suh-drin) (dextroamphetamine sulfate) SPANSULE® sustained-release capsules, CII - What is the most important information I should know about ...
-
PRINCIPAL DISPLAY PANEL - 5 mg BOTTLE LABEL

-
PRINCIPAL DISPLAY PANEL - 5 mg CARTON

-
PRINCIPAL DISPLAY PANEL - 10 mg BOTTLE LABEL

-
PRINCIPAL DISPLAY PANEL - 10 mg CARTON

-
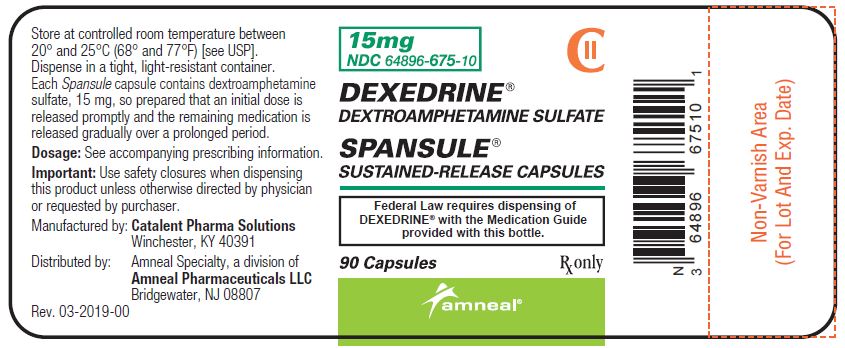
PRINCIPAL DISPLAY PANEL - 15 mg BOTTLE LABEL
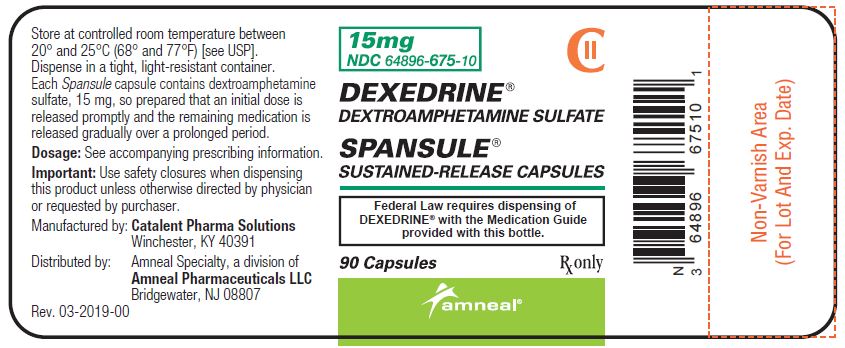
-
PRINCIPAL DISPLAY PANEL - 15 mg CARTON

-
INGREDIENTS AND APPEARANCEProduct Information