Label: DAPIPRAZOLE kit
- NDC Code(s): 53020-245-01, 53020-255-01, 53020-265-01
- Packager: Baradaina, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
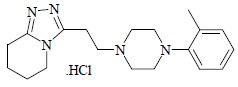
DESCRIPTION:For ophthalmic use only. Dapiprazole hydrochloride is an alpha-adrenergic blocking agent. Dapiprazole hydrochloride is 5,6,7,8-tetrahydro-3-[2-(4- o .tolyl-1-piperazinyl)ethyl] ...
-
CLINICAL PHARMACOLOGY:Dapiprazole hydrochloride ophthalmic solution acts through blocking the alpha-adrenergic receptors in smooth muscle. Dapiprazole hydrochloride ophthalmic solution produces miosis through an effect ...
-
INDICATIONS AND USAGE:Dapiprazole hydrochloride ophthalmic solution is indicated in the treatment of iatrogenically induced mydriasis produced by adrenergic (phenylephrine) or parasympatholytic (tropicamide) agents ...
-
CONTRAINDICATIONS:Miotics are contraindicated where constriction is undesirable; such as acute iritis, and in those subjects showing hypersensitivity to any component of this preparation.
-
WARNING:For Topical Ophthalmic Use Only. NOT FOR INJECTION. Do not touch the dropper up to lids or any surface, as this may contaminate the solution. Dapiprazole hydrochloride ophthalmic solution should ...
-
PRECAUTIONS:Information to Patients: Miosis may cause difficulty in dark adaptation and may reduce the field of vision. Patients should exercise caution when involved in night driving or other ...
-
ADVERSE REACTIONS:In controlled studies the most frequent reaction to dapiprazole was conjunctival injection lasting 20 minutes in over 80% of patients. Burning on instillation of dapiprazole hydrochloride ...
-
DOSAGE AND ADMINISTRATION:Two drops followed 5 minutes later by an additional 2 drops applied topically to the conjunctiva of each eye should be administered after the ophthalmic examination to reverse the diagnostic ...
-
HOW SUPPLIED:Dapiprazole hydrochloride ophthalmic solution, 0.5% - Sterile is supplied as an outer package (NDC 53020-265-01) containing: NDC 53020-255-01 single vial of dapiprazole hydrochloride (25 mg ...
-
Storage and Stability of Eyedrops:Once the ophthalmic solution has been reconstituted it may be stored at 20° - 25°C (68° - 77°F) [See USP Controlled Temperature] for 21 days. Discard any solution that is not clear and ...
-
PRINCIPAL DISPLAY-Container Label Sterile - Dapiprazole HCI - Ophthalmic Solution - 0.5% E Y E D R O P S - Rx only - For Use in the Eyes - 5 mL - (when diluent is added) ...
-
INGREDIENTS AND APPEARANCEProduct Information