Label: CYCLOMYDRIL- cyclopentolate hydrochloride and phenylephrine hydrochloride solution/ drops
- NDC Code(s): 0065-0359-02, 0065-0359-05
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
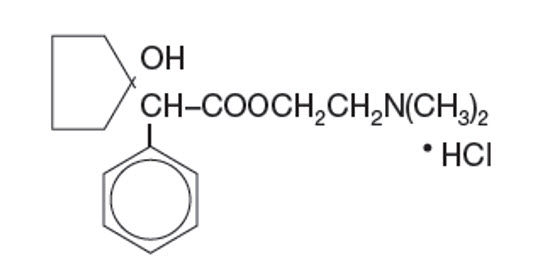
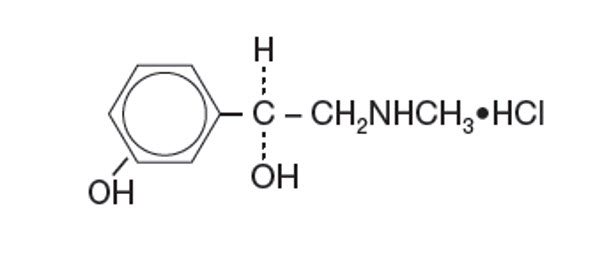
DESCRIPTIONCYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is a mydriatic prepared as a sterile topical ophthalmic solution. The active ingredients are ...
-
CLINICAL PHARMACOLOGYCyclopentolate hydrochloride is an anticholinergic drug and phenylephrine hydrochloride is an adrenergic drug. This combination induces mydriasis that is greater than that of either drug alone at ...
-
INDICATIONS AND USAGEFor the production of mydriasis.
-
CONTRAINDICATIONSDo not use in patients with untreated narrow-angle glaucoma or with untreated anatomically narrow angles or where there is hypersensitivity to any component of this preparation.
-
WARNINGSFOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. The use of this combination may have an adverse effect on individuals suffering from cardiovascular disease, hypertension, and hyperthyroidism ...
-
PRECAUTIONSGeneral - The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption. Caution should be observed when ...
-
ADVERSE REACTIONSOcular - The following ocular adverse experiences have been associated with the use of CYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution): increased ...
-
OVERDOSAGEExcessive dosage may produce behavioral disturbances, tachycardia, hyperpyrexia, hypertension, elevated intraocular pressure, vasodilation, urinary retention, diminished gastrointestinal motility ...
-
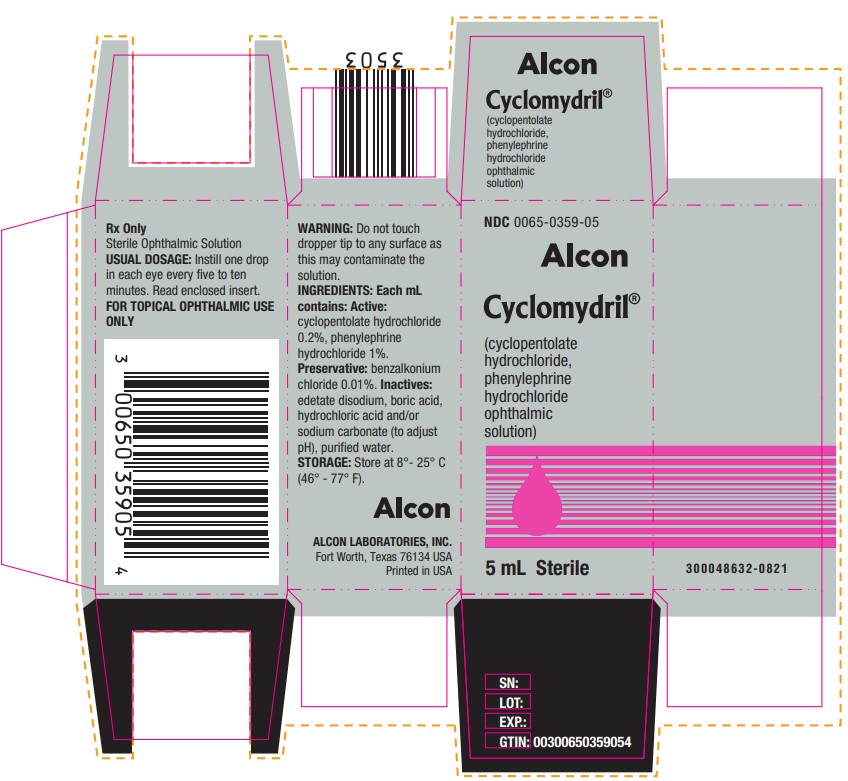
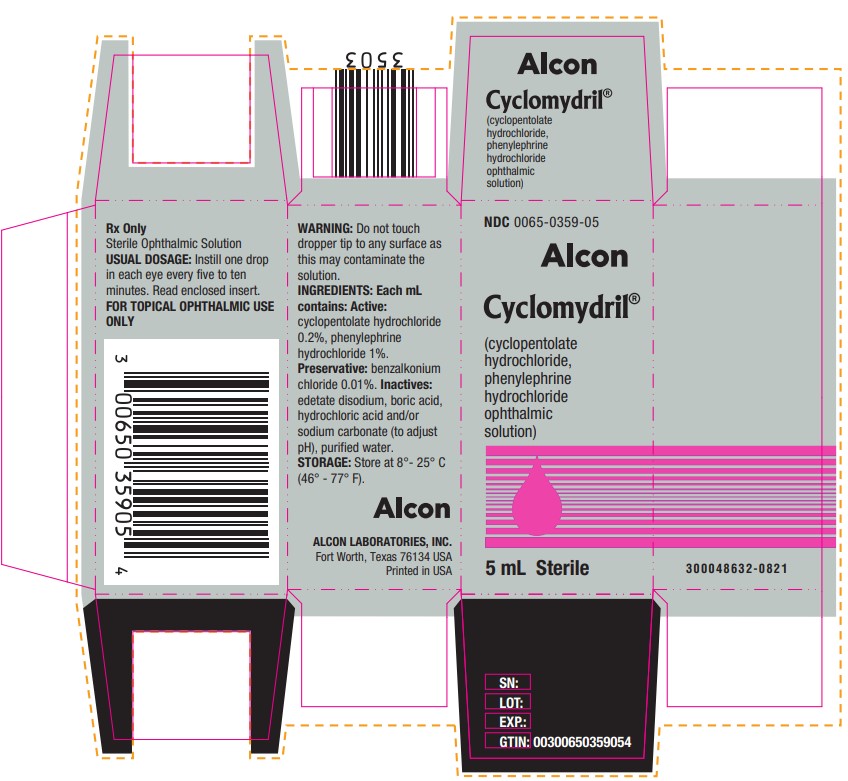
DOSAGE AND ADMINISTRATIONInstill one drop in each eye every five to ten minutes. To minimize systemic absorption, apply pressure over the nasolacrimal sac for two to three minutes following instillation. Observe infants ...
-
HOW SUPPLIEDCYCLOMYDRIL® (cyclopentolate hydrochloride and phenylephrine hydrochloride ophthalmic solution) is supplied as a sterile solution in 2 mL and 5 mL, in plastic DROP-TAINER® dispensers. 2 ...
-
SPL UNCLASSIFIED SECTIONDistributed by: ALCON LABORATORIES, INC. 6201 South Freeway - Fort Worth, Texas 76134-2099 - ©2004, 2013, 2018, 2021 Alcon Inc. T2018-97 - Revised: June 2018
-
PRINCIPAL DISPLAY PANELNDC 0065-0359-05 - Alcon - Cyclomydril® (cyclopentolate - hydrochloride, phenylephrine - hydrochloride - ophthalmic - solution) 5 mL Sterile - Rx Only - Sterile Ophthalmic Solution - USUAL ...
-
INGREDIENTS AND APPEARANCEProduct Information