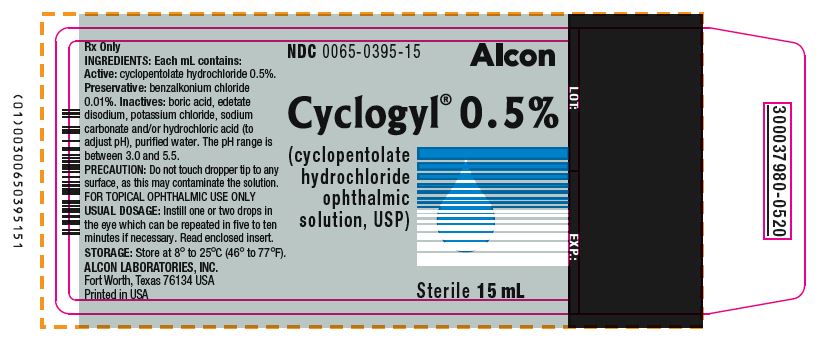
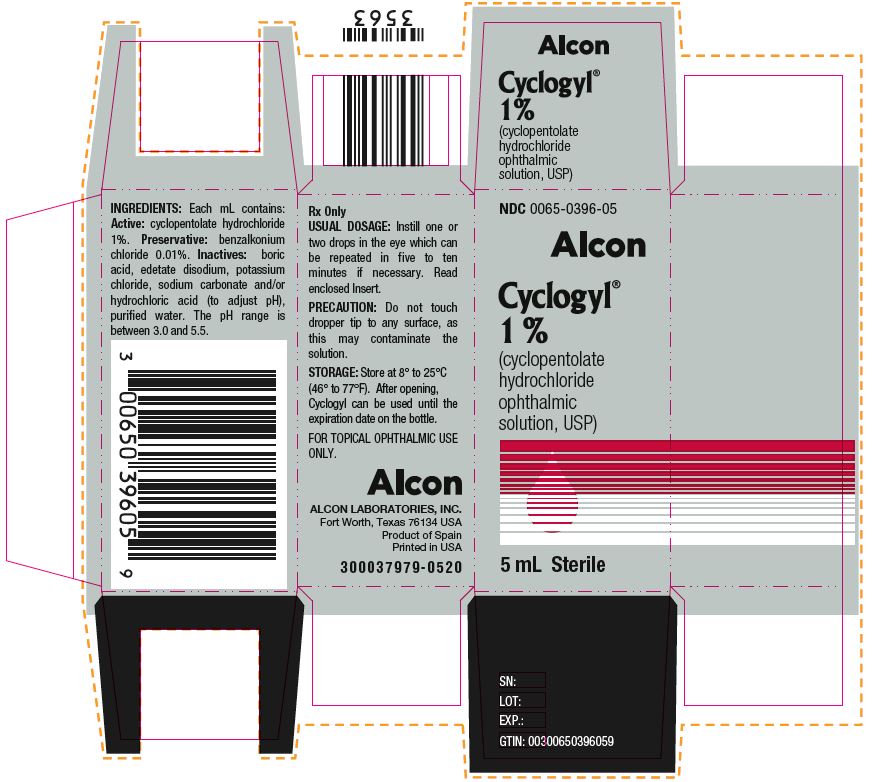
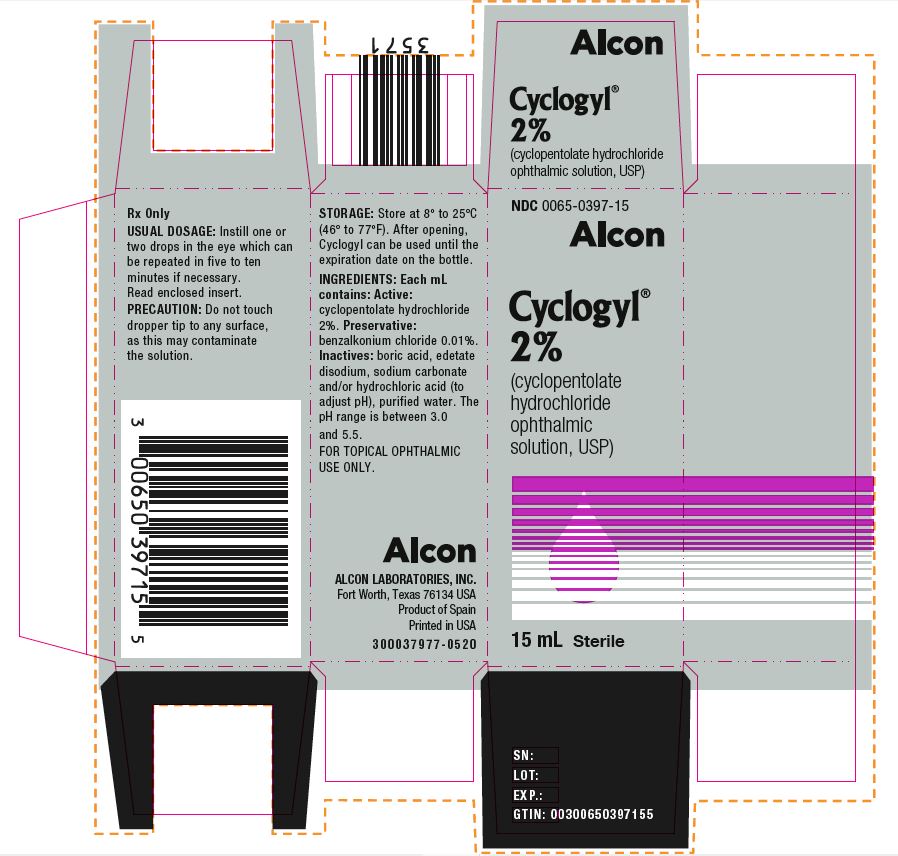
Label: CYCLOGYL- cyclopentolate hydrochloride solution/ drops
- NDC Code(s): 0065-0395-15, 0065-0396-02, 0065-0396-05, 0065-0396-15, view more
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Rx Only
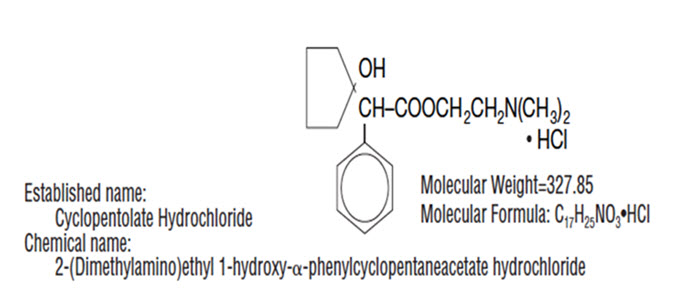
DESCRIPTION: CYCLOGYL® (cyclopentolate hydrochloride ophthalmic solution, USP) is an anticholinergic prepared as a sterile, borate buffered, solution for topical ocular use. It is supplied in ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation ...
-
INDICATIONS & USAGEINDICATIONS AND USAGE: Cyclopentolate hydrochloride is used to produce mydriasis and cycloplegia.
-
CONTRAINDICATIONSCONTRAINDICATIONS: Should not be used if the patient is hypersensitive to any component of this preparation.
-
WARNINGSWARNINGS: FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. This preparation may cause Central Nervous System (CNS) disturbances. This is especially true in younger age groups, but may occur at ...
-
PRECAUTIONS:General: The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption. Caution should be observed when ...
-
ADVERSE REACTIONS:Ocular: Increased intraocular pressure, burning, photophobia, blurred vision, irritation, hyperemia, conjunctivitis, blepharoconjunctivitis, punctate keratitis, synechiae have been ...
-
OVERDOSAGEOVERDOSAGE: Excessive dosage may produce behavioral disturbances, tachycardia, hyperpyrexia, hypertension, elevated intraocular pressure, vasodilation, urinary retention, diminished ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Adults: Instill one or two drops of 0.5%, 1% or 2% solution in the eye which may be repeated in five to ten minutes if necessary. Complete recovery usually occurs in 24 ...
-
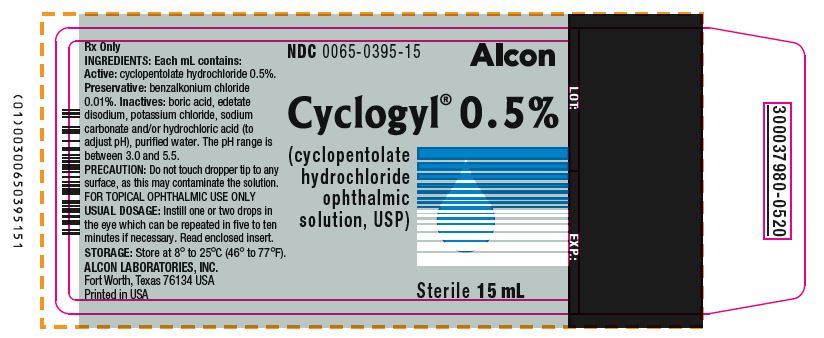
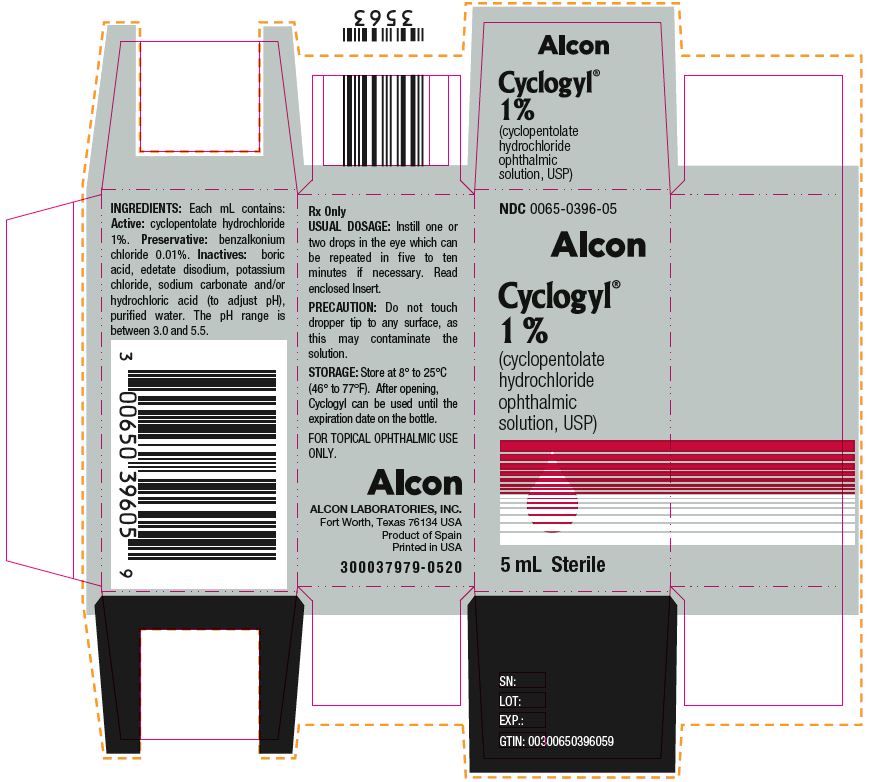
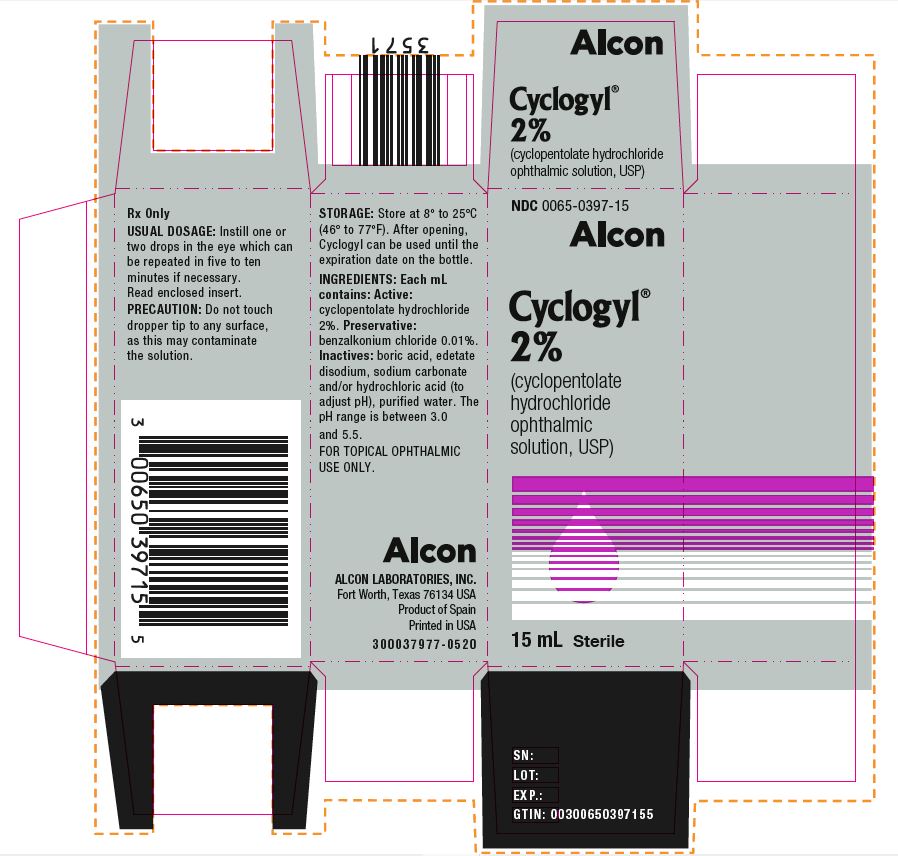
HOW SUPPLIEDHOW SUPPLIED: In multiple-dose plastic DROP-TAINER® dispensers: 0.5% CYCLOGYL 1% CYCLOGYL 2% CYCLOGYL - 15 mL NDC 0065-0395-15 2 mL NDC ...
-
SPL UNCLASSIFIED SECTION© 2021 Alcon Inc. Revised: November 2021 - ALCON LABORATORIES, INC. Fort Worth, Texas 76134 - 300051372-1121
-
PRINCIPAL DISPLAY PANELNDC 0065-0395-15 - Alcon - Cyclogyl® 0.5% (cyclopentolate hydrochloride ophthalmic solution, USP) 15 mL Sterile - INGREDIENTS: Each mL contains: Active: cyclopentolate hydrochloride ...
-
INGREDIENTS AND APPEARANCEProduct Information