Label: CUVPOSA- glycopyrrolate liquid
- NDC Code(s): 0259-0501-16
- Packager: Merz Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CUVPOSA® safely and effectively. See full prescribing information for CUVPOSA. CUVPOSA (glycopyrrolate) oral solution - Initial ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECUVPOSA is indicated to reduce chronic severe drooling in patients aged 3 to 16 years with neurologic conditions associated with problem drooling (e.g., cerebral palsy).
-
2 DOSAGE AND ADMINISTRATIONCUVPOSA must be measured and administered with an accurate measuring device [see Patient Counseling Information (17)]. Initiate dosing at 0.02 mg/kg orally three times daily and titrate in ...
-
3 DOSAGE FORMS AND STRENGTHSCUVPOSA is available as a 1mg/5 mL clear, cherry-flavored solution for oral administration in 16 ounce bottles.
-
4 CONTRAINDICATIONSCUVPOSA is contraindicated in: Patients with medical conditions that preclude anticholinergic therapy (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ...
-
5 WARNINGS AND PRECAUTIONS5.1 Constipation or Intestinal Pseudo-obstruction - Constipation is a common dose-limiting adverse reaction which sometimes leads to glycopyrrolate discontinuation [see Adverse Reactions (6.1)] ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: Constipation or intestinal pseudo-obstruction [see Warnings and Precautions (5.1)] Incomplete mechanical ...
-
7 DRUG INTERACTIONSDrugs Affected by Reduced GI Transit Time - Glycopyrrolate reduces GI transit time, which may result in altered release of certain drugs when formulated in delayed- or controlled-release dosage ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data in pregnant women for Cuvposa to inform decisions concerning any drug-associated risks. In pregnant rats, daily oral administration ...
-
10 OVERDOSAGEBecause glycopyrrolate is a quaternary amine which does not easily cross the blood-brain barrier, symptoms of glycopyrrolate overdosage are generally more peripheral in nature rather than central ...
-
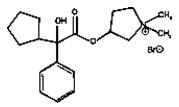
11 DESCRIPTIONCUVPOSA is an anticholinergic drug available as an oral solution containing 1 mg glycopyrrolate per 5 mL. The chemical name for glycopyrrolate is pyrrolidinium, 3-[(cyclopentylhydroxyphenylacetyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Glycopyrrolate is a competitive inhibitor of acetylcholine receptors that are located on certain peripheral tissues, including salivary glands. Glycopyrrolate ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - When glycopyrrolate was administered via oral gavage to mice for up to 24 months at dosages of 2.5, 7, and 20 mg/kg/day in both ...
-
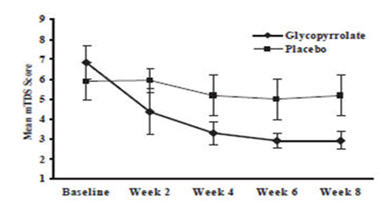
14 CLINICAL STUDIESCUVPOSA was evaluated in a multi-center, randomized, double-blind, placebo-controlled, parallel, eight-week study for the control of pathologic drooling in children (Study 1). The study enrolled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNDC 0259-0501-16; 1 mg/5mL clear, cherry-flavored solution; 16 oz. bottle. Store at room temperature 20° - 25°C (68° - 77°F); excursions permitted to 15° - 30°C (59° - 86°F) [See USP Controlled ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information) Advise patients/caregivers to measure CUVPOSA with an accurate measuring device. A household teaspoon is not an accurate ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Mikart, LLC - Atlanta, GA 30318 - Manufactured for: Merz Pharmaceuticals, LLC - Raleigh, NC 27615 - © 2023 Merz Pharmaceuticals, LLC - CUVPOSA® is a registered trademark of Merz ...
-
PATIENT PACKAGE INSERTPATIENT and CAREGIVER INFORMATION - CUVPOSA (glycopyrrolate) Oral Solution - Please read the Patient and Caregiver Information that comes with CUVPOSA before you start giving it to your child, and ...
-
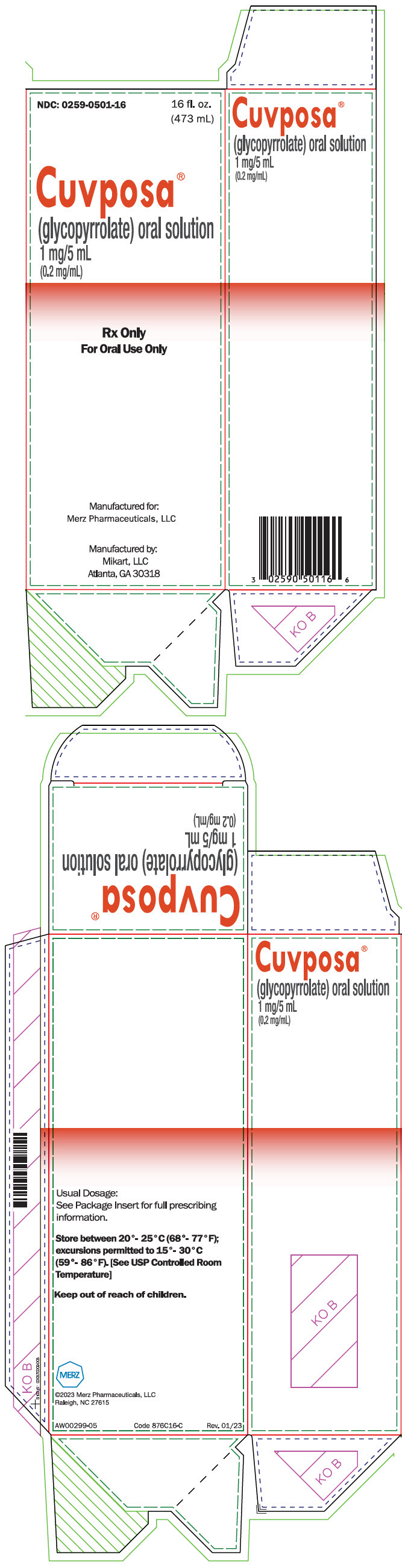
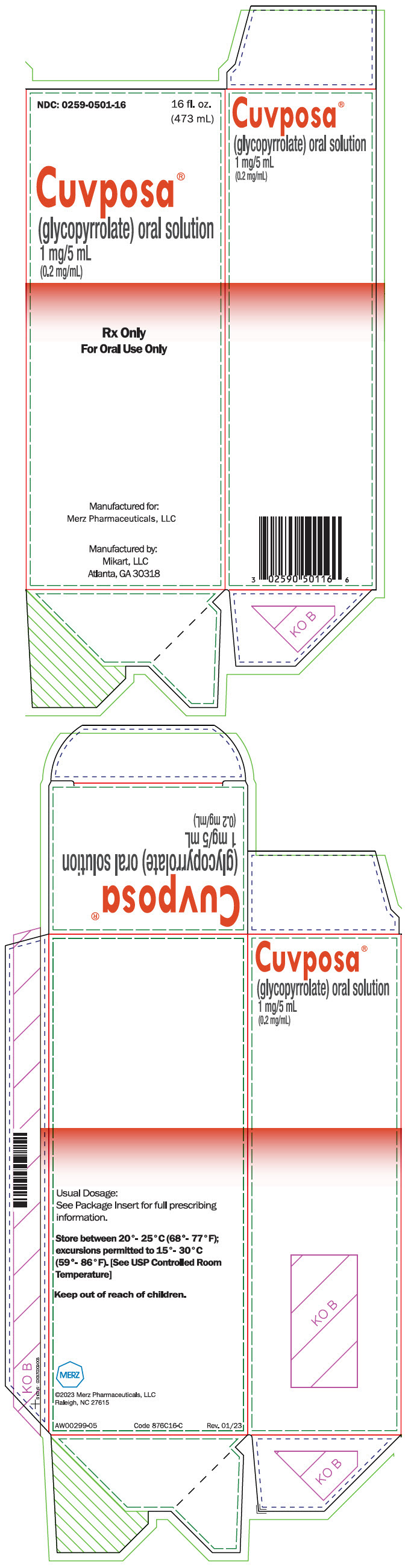
PRINCIPAL DISPLAY PANEL - 473 mL Bottle CartonNDC: 0259-0501-16 - 16 fl. oz. (473 mL) Cuvposa® (glycopyrrolate) oral solution - 1 mg/5 mL - (0.2 mg/mL) Rx Only - For Oral Use Only - Manufactured for: Merz Pharmaceuticals, LLC - Manufactured by: Mikart ...
-
INGREDIENTS AND APPEARANCEProduct Information