Label: CUROSURF- poractant alfa suspension
- NDC Code(s): 10122-510-01, 10122-510-03
- Packager: Chiesi USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated May 2, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CUROSURF® safely and effectively. See full prescribing information for CUROSURF. CUROSURF (poractant alfa) intratracheal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
CUROSURF® (poractant alfa) Intratracheal Suspension is indicated for the rescue treatment of Respiratory Distress Syndrome (RDS) in premature infants. CUROSURF reduces mortality and ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions - For intratracheal administration only. CUROSURF should be administered by, or under the supervision of clinicians experienced in intubation ...
-
3 DOSAGE FORMS AND STRENGTHS
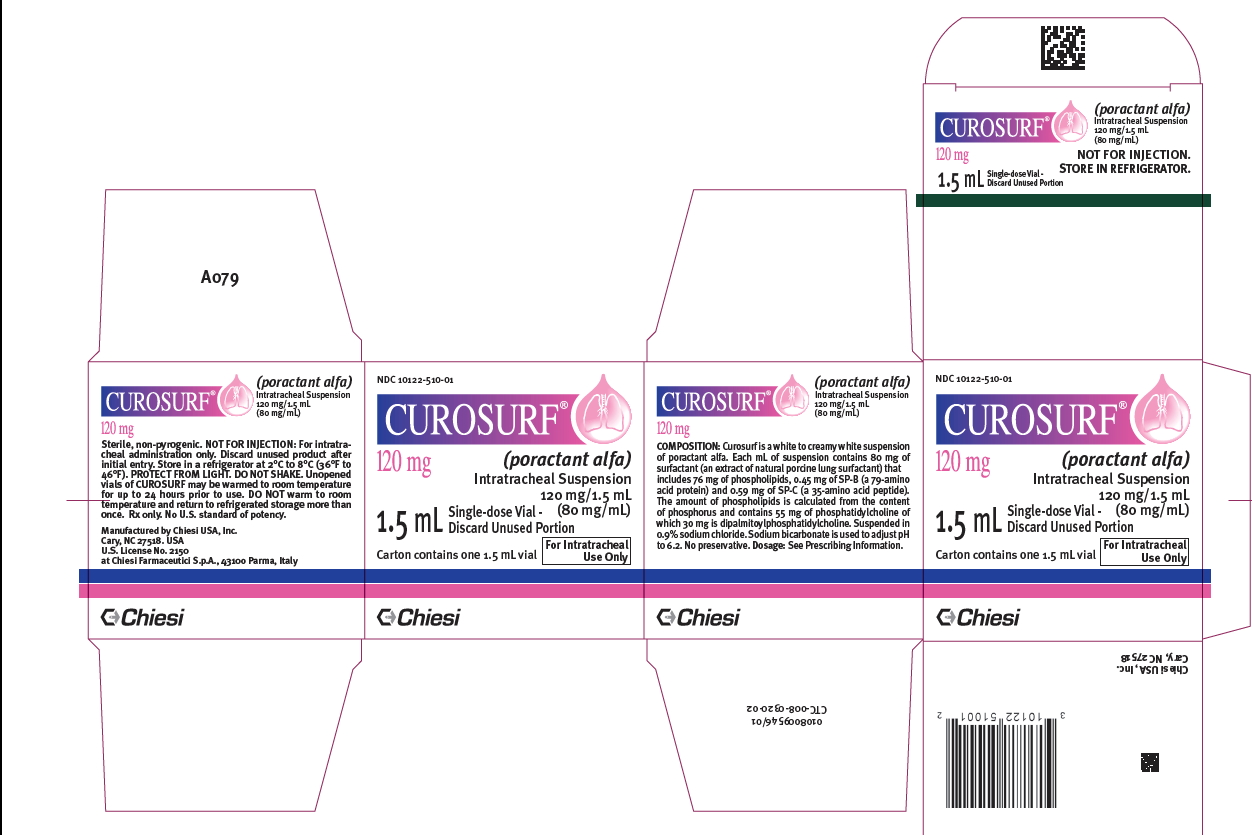
Intratracheal suspension: CUROSURF (poractant alfa) is a white to creamy white suspension available in: 120 mg/1.5 mL (80 mg/mL) single-dose vials - 240 mg/3 mL (80 mg/mL) single-dose vials
-
4 CONTRAINDICATIONS
None.
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Changes in Oxygenation and Lung Compliance - The administration of exogenous surfactants, including CUROSURF, can rapidly affect oxygenation and lung compliance. Therefore, infants ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use - The safety and effectiveness of CUROSURF for the rescue treatment, including the reduction of mortality and pneumothoraces, of Respiratory Distress Syndrome (RDS) have been ...
-
10 OVERDOSAGE
There have been no reports of overdosage following the administration of CUROSURF. In the event of accidental overdosage, and if there are clear clinical effects on the infant's respiration ...
-
11 DESCRIPTION
Poractant alfa is an extract of natural porcine lung (pulmonary) surfactant consisting of 99% polar lipids (mainly phospholipids) and 1% hydrophobic low molecular weight surfactant associated ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - Endogenous pulmonary surfactant reduces surface tension at the air-liquid interface of the alveoli during ventilation and stabilizes the alveoli against collapse at ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies to assess potential carcinogenic effects of CUROSURF have not been conducted. Poractant alfa was negative for genotoxicity ...
-
14 CLINICAL STUDIES
14.1 Rescue Treatment of Respiratory Distress Syndrome - The clinical efficacy of CUROSURF in the treatment of established Respiratory Distress Syndrome (RDS) in premature infants was ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CUROSURF (poractant alfa) intratracheal suspension is a white to creamy white suspension available in sterile, rubber-stoppered clear glass vials containing (one vial per carton): 120 mg/1.5 mL ...
-
PRINCIPAL DISPLAY PANEL

-
PRINCIPAL DISPLAY PANEL
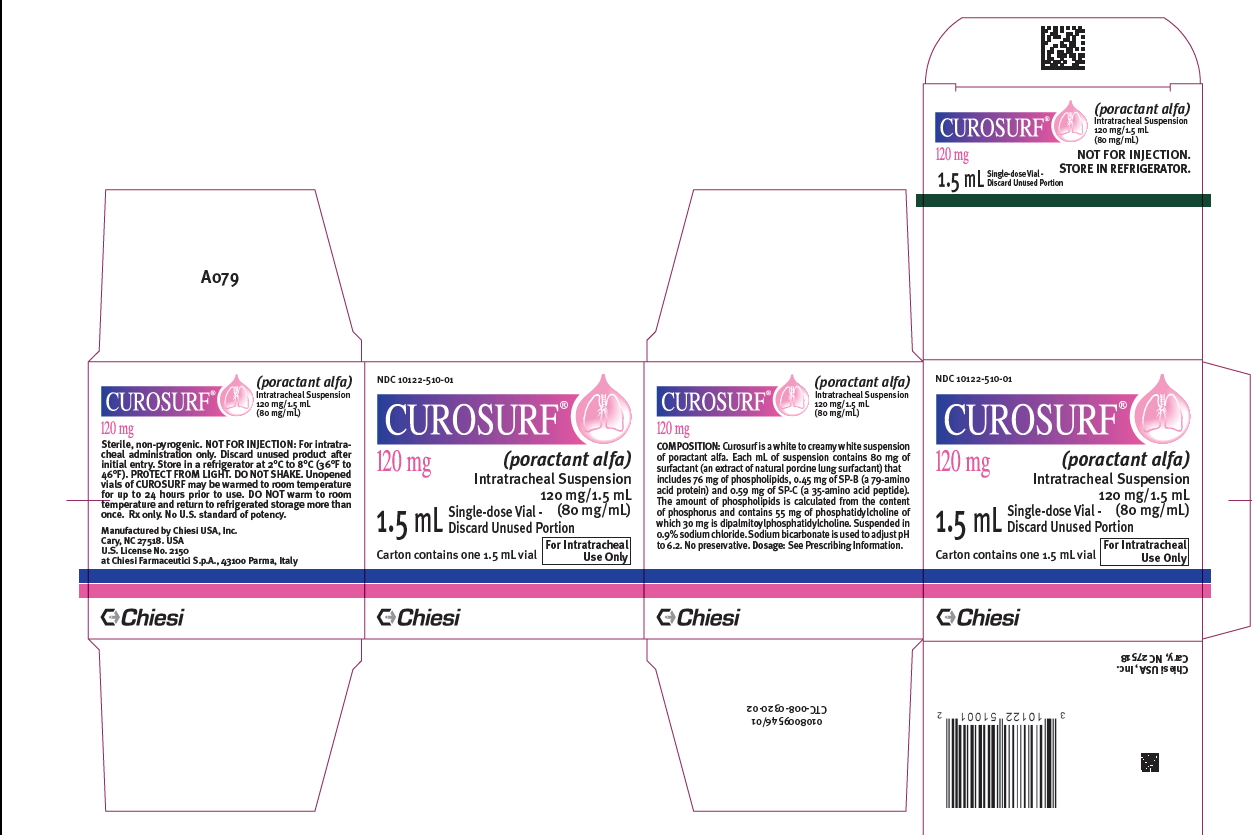
-
INGREDIENTS AND APPEARANCEProduct Information