Label: SPHERUSOL- coccidioides immitis spherule-derived skin test antigen injection, solution
- NDC Code(s): 59584-140-01
- Packager: Nielsen BioSciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Spherusol - ® safely and effectively. See full prescribing information for Spherusol - ®. Coccidioides immitis Spherule-Derived ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING
- The expected response to Spherusol ® is a local area of inflammation at the site of the skin test. The reaction is usually dime to quarter size reaching maximum diameter between 24 and 48 hours. Larger accelerated reactions can occur, which may require treatment with local cold compresses and anti-inflammatory medication. ( 2.3, 6.1)
- Systemic reactions can occur with skin test antigens and in certain individuals these reactions may be life-threatening or cause death. Emergency measures and personnel trained in their use should be immediately available. Patients should be observed for at least 20 minutes following the administration of a skin test. ( 6.2)
- Spherusol ® should never be given intravenously. ( 5)
- To report SUSPECTED ADVERSE REACTIONS, contact Nielsen BioSciences, Inc. at (855) 855-1212 or MEDWATCH, Food and Drug Administration (FDA), 5600 Fishers Lane, Rockville, MD 20852-9782. Telephone: (800) 332-1088 or www.vaers.hhs.gov. ( 6.2)
-
1 INDICATIONS AND USAGESpherusol - ® is a skin test antigen indicated for the detection of delayed-type hypersensitivity to - Coccidioides immitis in individuals with a history of pulmonary ...
-
2 DOSAGE AND ADMINISTRATION2.1 Preparation for Administration - Spherusol - ® is a clear, colorless sterile solution for intradermal administration. Parenteral drug products should be inspected ...
-
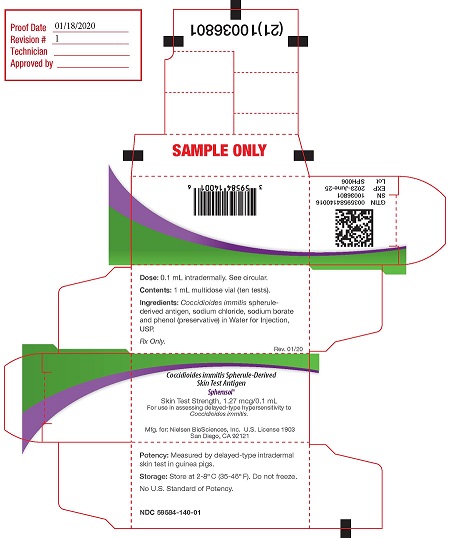
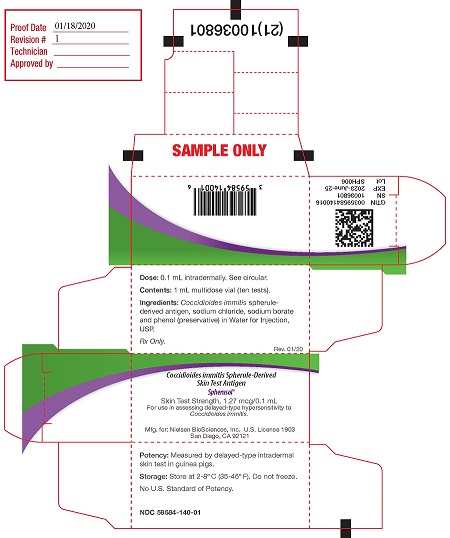
3 DOSAGE FORMS AND STRENGTHSSpherusol - ® is a solution for intradermal injection supplied in a 1 mL multi-dose vial. Each 0.1 mL dose contains 1.27 mcg of spherule-derived - Coccidioides immitis ...
-
4 CONTRAINDICATIONSA severe allergic reaction (e.g., anaphylaxis) to Spherusol - ®, or any component of Spherusol - ® or other coccidioidin products is a contraindication to administration ...
-
5 WARNINGS AND PRECAUTIONS5.1 Prevention and Management of Acute Hypersensitivity Reactions - Prior to administration, the healthcare provider should review the medical history for possible skin test sensitivity and ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a skin test antigen cannot be ...
-
7 DRUG INTERACTIONS7.1 Corticosteroids and Immunosuppressives - Corticosteroids and Immunosuppressive agents may suppress the response to the skin test. Pharmacologic doses of corticosteroids may suppress the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - The safety and effectiveness of Spherusol - ® in pregnant women have not been established. Pregnancy Category C - Animal reproduction studies have not been ...
-
11 DESCRIPTIONSpherusol - ® is a sterile aqueous solution of extracts of - C. immitis spherules. The multi-dose vial contains 0.9% sodium chloride and 0.014% sodium borate with 0.4% phenol ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In individuals with a history of pulmonary coccidioidomycosis Spherusol - ® is thought to elicit a cellular immune reaction to - C. immitis ...
-
14 CLINICAL STUDIESThe delayed-type hypersensitivity response following administration of Spherusol - ® was evaluated in one U.S. study which enrolled persons with a history of coccidioidomycosis. Two ...
-
15 REFERENCESEdwards, PQ and Palmer CE. Prevalence of sensitivity to coccodioidin, with special reference to specific and nonspecific reactions to coccidioidin. Dis Chest. 31:35. 35-60, 1957. Emmons, C.W. ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSpherusol - ® is available in 1 mL multidose vial. NDC# 59584-140-01: multi-dose vial. Store refrigerated at 2° to 8°C (35° to 46°F). Do not freeze. Discard if frozen. Do not ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be: Informed of the potential benefits and risks of skin testing with Spherusol - ®. Instructed to report any adverse events to their healthcare ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1 mL Vial - NDC 59584-140-01 - Coccidioides immitis Spherule-Derived Skin Test Antigen - Spherusol ® Vol:1mL (10 Tests) Dose:0.1mL Intradermally - See ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1 mL Carton - Dose: 0.1 mL intradermally. See circular. (1st panel) Contents: 1 mL multidose vial (10 tests) Ingredients: Coccidioides immitis ...
-
INGREDIENTS AND APPEARANCEProduct Information