Label: CLOROTEKAL- chloroprocaine hydrochloride injection, solution
- NDC Code(s): 0264-7055-05, 0264-7055-10
- Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 4, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLOROTEKAL® safely and effectively. See full prescribing information for CLOROTEKAL®. CLOROTEKAL® (chloroprocaine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECLOROTEKAL® (chloroprocaine hydrochloride) is indicated for intrathecal injection for the production of subarachnoid block (spinal anesthesia) in adults undergoing surgical procedures. Indicated ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Information - CLOROTEKAL® must only be administered by clinicians with the necessary knowledge and experience in the intrathecal anesthesia administration ...
-
3 DOSAGE FORMS AND STRENGTHSCLOROTEKAL® is supplied as a single-dose sterile, clear, colorless solution in a Type I (USP) glass ampule that provides 50 mg of chloroprocaine hydrochloride in 5 mL aqueous solution ...
-
4 CONTRAINDICATIONSCLOROTEKAL® is contraindicated in patients with a known hypersensitivity to the active substance, medicinal products of the PABA (para-aminobenzoic acid) ester group, other ester-type local ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risks with Neuraxial Administration - Local anesthetics should only be administered by clinicians who are well versed in diagnosis and management of dose-related toxicity and other acute ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described, or described in greater detail, in other sections: Cardiovascular System Reactions [see Warnings and Precautions (5.2)] Central Nervous ...
-
7 DRUG INTERACTIONSConcurrent administration of vasopressor drugs (for the treatment of hypotension related to obstetric blocks) and ergot-type oxytocic drugs may cause severe, persistent hypertension or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The limited available data with chloroprocaine use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. There ...
-
10 OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use or to underventilation secondary to upward extension of spinal anesthesia ...
-
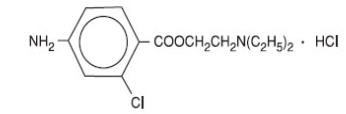
11 DESCRIPTIONCLOROTEKAL® is a sterile non pyrogenic local anesthetic. The active ingredient in CLOROTEKAL® is chloroprocaine hydrochloride (benzoic acid, 4-amino-2-chloro-2-(diethylamino) ethyl ester ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Chloroprocaine, like other local anesthetics, blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate carcinogenic potential of chloroprocaine have not been ...
-
14 CLINICAL STUDIES14.1 Study 1 - A Phase 2 single-center, prospective, randomized, observer-blind study evaluated the efficacy and the tolerability of chloroprocaine 30, 40, and 50 mg after spinal injection in 45 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGThe CLOROTEKAL® is supplied as a 50mg/5mL (10 mg/mL) equivalent to 44.05 mg/5 mL (8.81 mg/mL) chloroprocaine Type I glass ampules, stored in cartons containing 10 single-dose ...
-
17 PATIENT COUNSELING INFORMATIONInform patients in advance that chloroprocaine-containing products can cause temporary loss of sensation or motor activity, usually in the lower half of the body, following proper administration ...
-
SPL UNCLASSIFIED SECTIONRx only - Clorotekal® is a registered trademark of Sintetica S.A. Manufactured by: Sintetica S.A. Switzerland - Manufactured for: B. Braun Medical Inc. Bethlehem, PA 18018-3524 ...
-
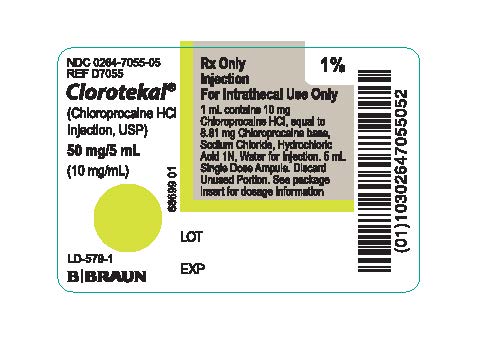
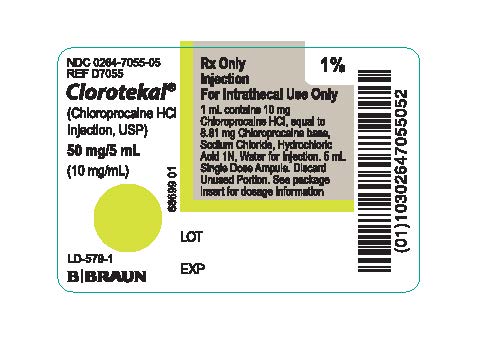
PRINCIPAL DISPLAY PANEL - 5 mL Ampule LabelNDC 0264-7055-05 - REF D7055 - Clorotekal® (Chloroprocaine HCl - Injection, USP) 50 mg/5 mL - (10 mg/mL) LD-579-1 - Rx Only - Injection - For Intrathecal Use Only - 1 mL contains 10 mg Chloroprocaine ...
-
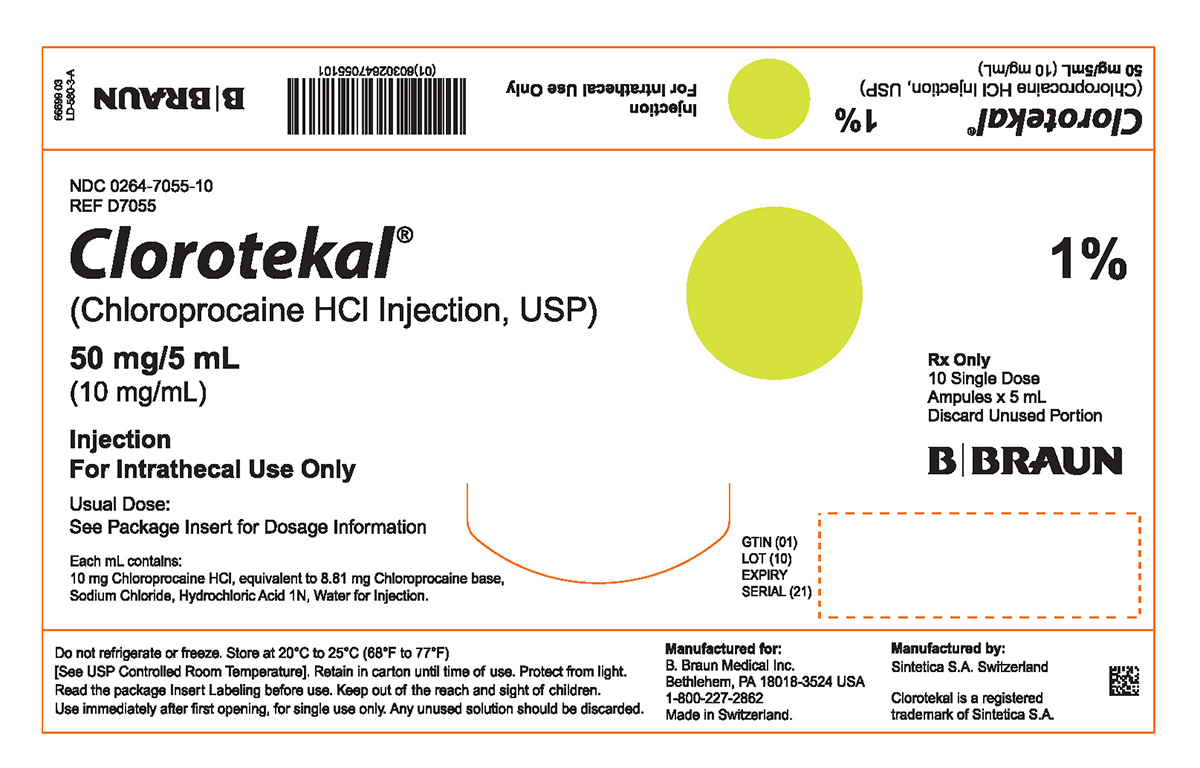
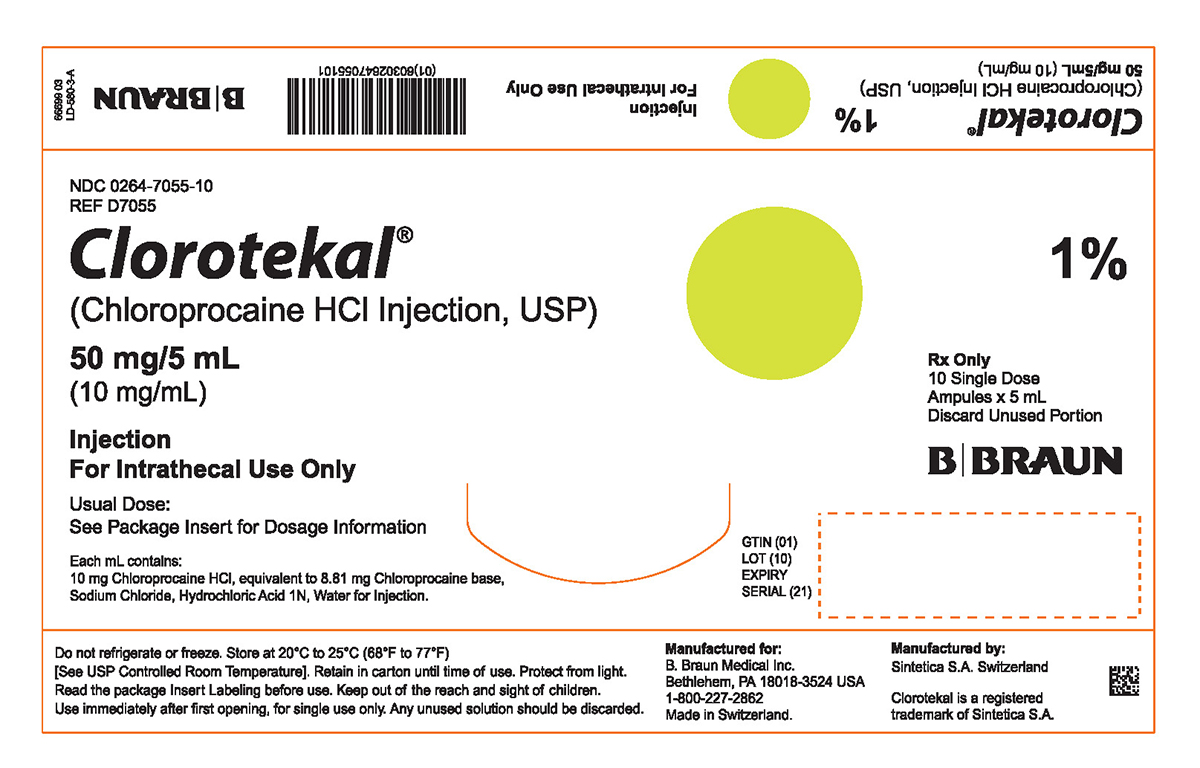
PRINCIPAL DISPLAY PANEL - 5 mL Ampule Carton (A)NDC 0264-7055-10 - REF D7055 - Clorotekal® (Chloroprocaine HCl Injection, USP) 50 mg/5 mL - (10 mg/mL) Injection - For Intrathecal Use Only - Usual Dose: See Package Insert for Dosage Information ...
-
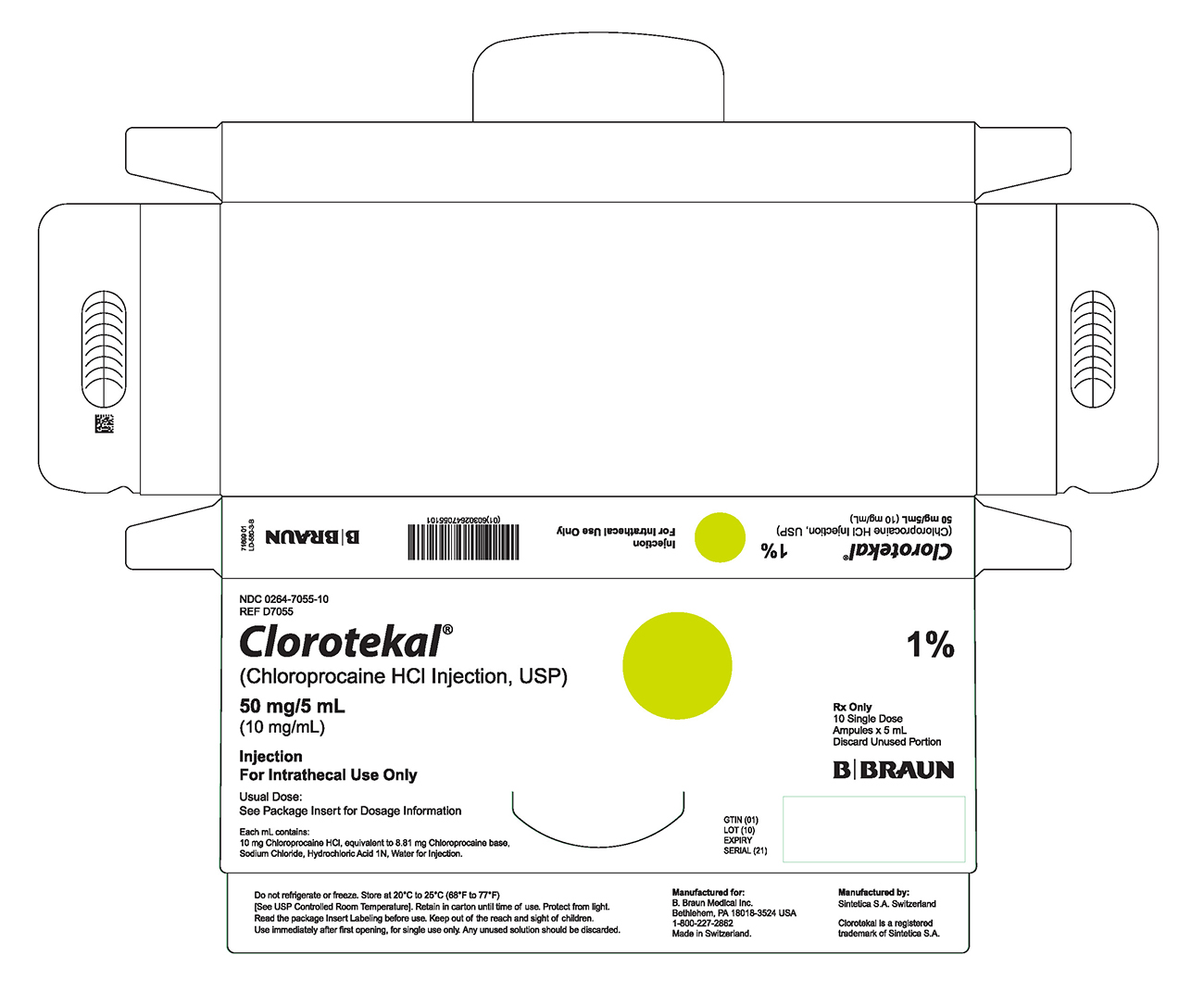
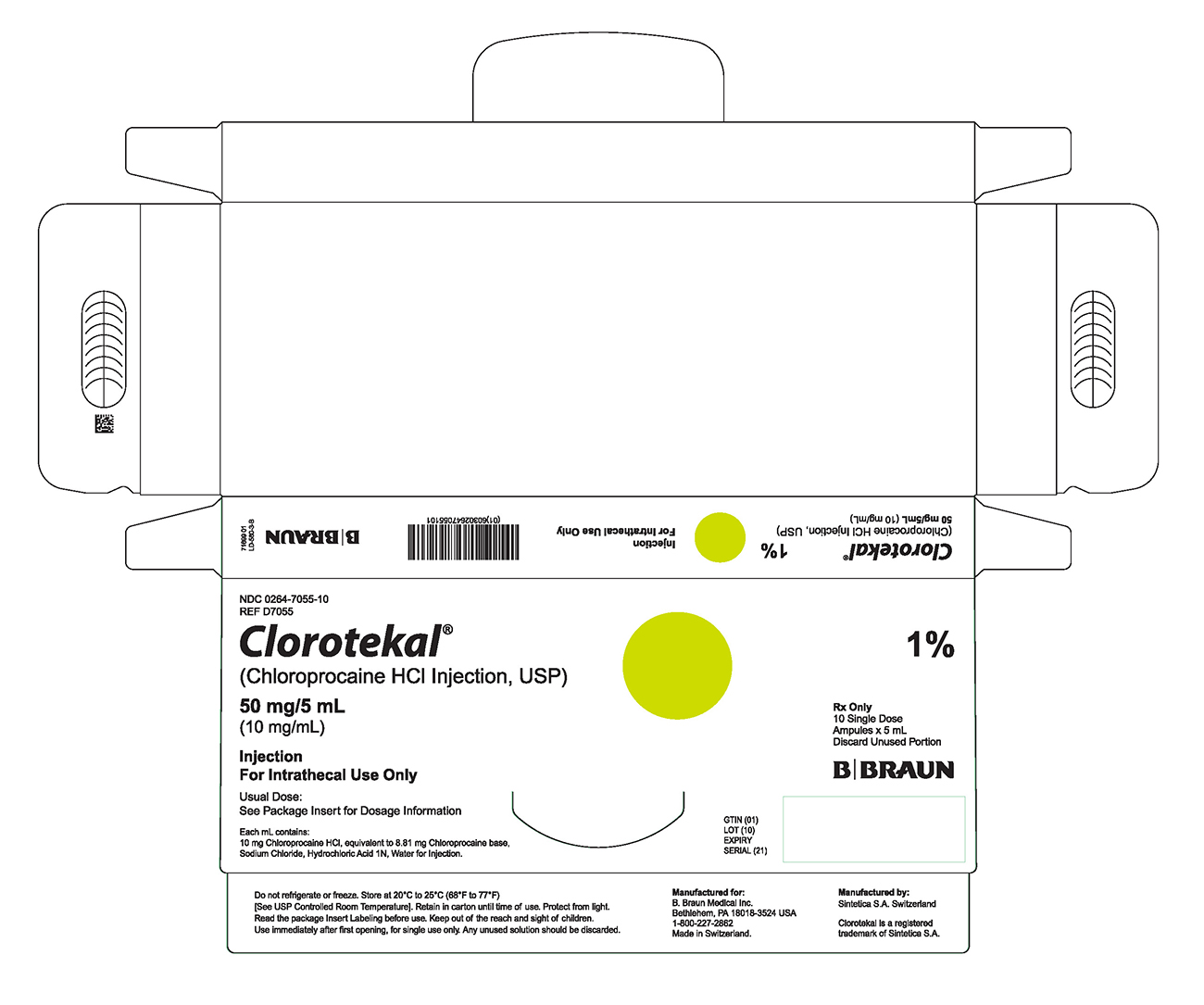
PRINCIPAL DISPLAY PANEL - 5 mL Ampule Carton (B)NDC 0264-7055-10 - REF D7055 - Clorotekal® (Chloroprocaine HCl Injection, USP) 50 mg/5 mL - (10 mg/mL) Injection - For Intrathecal Use Only - Usual Dose: See Package Insert for Dosage Information ...
-
INGREDIENTS AND APPEARANCEProduct Information