Label: CLODAN- clobetasol propionate shampoo
- NDC Code(s): 43538-950-04
- Packager: Medimetriks Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Clodan® (clobetasol propionate) Shampoo, 0.05% safely and effectively. See full prescribing information for Clodan® (clobetasol ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Indication - Clodan® (clobetasol propionate) Shampoo, 0.05% is a super-high potent topical corticosteroid formulation indicated for the treatment of moderate to severe forms of scalp ...
-
2 DOSAGE AND ADMINISTRATIONClodan® (clobetasol propionate) Shampoo, 0.05% is for topical use only, and not for ophthalmic, oral or intravaginal use. Clodan® (clobetasol propionate) Shampoo, 0.05% should be applied onto dry ...
-
3 DOSAGE FORMS AND STRENGTHSShampoo, 0.05%, w/w. Each gram of Clodan® (clobetasol propionate) Shampoo, 0.05% contains 0.5 mg of clobetasol propionate in a translucent, colorless viscous liquid.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Effects on the Endocrine System - Clobetasol propionate is a highly potent topical corticosteroid that has been shown to suppress the HPA axis at the lowest doses tested. Systemic absorption ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy: Teratogenic effects: Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. Therefore, clobetasol propionate shampoo, 0.05% should be ...
-
10 OVERDOSAGETopically applied, Clodan® (clobetasol propionate) Shampoo, 0.05% can be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1)]
-
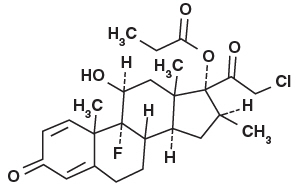
11 DESCRIPTIONClodan® (clobetasol propionate) Shampoo, 0.05% contains clobetasol propionate, a synthetic fluorinated corticosteroid, for topical use. The corticosteroids constitute a class of primarily ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Like other topical corticosteroids, Clodan® (clobetasol propionate) Shampoo, 0.05% has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Clobetasol propionate was not carcinogenic to rats when topically applied for 2 years at concentrations up to 0.005% which corresponded ...
-
14 CLINICAL STUDIESThe safety and efficacy of clobetasol propionate shampoo, 0.05% have been evaluated in two clinical trials involving 290 subjects with moderate to severe scalp psoriasis. In both trials, subjects ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGClodan® (clobetasol propionate) Shampoo, 0.05% is a translucent, colorless viscous liquid, supplied in 4 fl. oz. (118 mL) bottles. NDC 43538-950-04 - Storage: Keep bottle tightly closed. Store ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient Information) Information for Patients - Inform the patient using topical corticosteroids to adhere to the following instructions: This medication is ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Medimetriks Pharmaceuticals, Inc. 383 Route 46 West, Fairfield, NJ 07004-2402 • www.medimetriks.com - Manufactured by: Padagis, Yeruham, Israel - Rev. 05/2022 - IP035-R3
-
Patient InformationClodan® (clobetasol propionate) Shampoo, 0.05% Important: For use on the scalp only. Do not get Clodan® (clobetasol propionate) Shampoo, 0.05% near or in your eyes, mouth or vagina. Read ...
-
Instructions for UseClodan® (clobetasol propionate) Shampoo, 0.05% Important: For use on the scalp only. Do not get Clodan® (clobetasol propionate) Shampoo, 0.05% near or in your eyes, mouth or vagina. Read ...
-
PRINCIPAL DISPLAY PANEL - 118 mL Bottle LabelNDC 43538-950-04 - RX Only - Clodan® (clobetasol propionate) Shampoo, 0.05% For External Use Only - Do Not Use in Eyes - 4 FL. OZ. (118 mL) MEDIMETRIKS - PHARMACEUTICALS, INC. 6A926 EK F2
-
INGREDIENTS AND APPEARANCEProduct Information