Label: CLINDESSE- clindamycin phosphate cream
- NDC Code(s): 45802-042-01, 45802-042-02
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 30, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLINDESSE safely and effectively. See full prescribing information for CLINDESSE. CLINDESSE® (clindamycin phosphate) vaginal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Treatment of Bacterial Vaginosis - Clindesse is indicated for the treatment of bacterial vaginosis (formerly referred to as Haemophilus vaginitis, Gardnerella vaginitis, nonspecific ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose is the complete contents of a single pre-filled applicator containing 5 g of Clindesse cream administered once intravaginally at any time of the day. Not for ophthalmic ...
-
3 DOSAGE FORMS AND STRENGTHSClindesse is an intravaginal cream containing clindamycin phosphate 2%. Each pre-filled, single-dose applicator delivers approximately 5 g of cream containing approximately 100 mg of ...
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Clindesse is contraindicated in individuals with a history of hypersensitivity to clindamycin or other lincosamides. Reported reactions to other formulations of ...
-
5 WARNINGS AND PRECAUTIONS5.1 Clostridioides difficile-Associated Diarrhea (CDAD) Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ...
-
6 ADVERSE REACTIONS6.1 Clinical Study Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared ...
-
7 DRUG INTERACTIONSNo formal drug interaction studies have been conducted for Clindesse. 7.1 Neuromuscular Blocking Agents - Orally or intravenously administered clindamycin has neuromuscular blocking properties ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Clindesse has not been studied in pregnant women. The systemic exposure (based on AUC and Cmax) of Clindesse administered intravaginally is substantially lower than ...
-
10 OVERDOSAGEVaginally applied clindamycin phosphate vaginal cream 2% could be absorbed in sufficient amounts to produce systemic effects [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
-
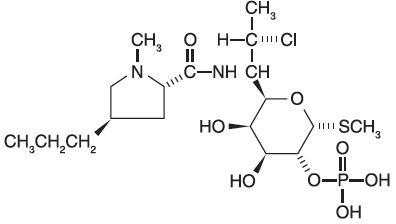
11 DESCRIPTIONClindamycin phosphate, a lincosamide, is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clindamycin is an antibacterial drug [see Clinical Pharmacology, Microbiology (12.4)]. 12.3 Pharmacokinetics - Following a single intravaginal application of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed ...
-
14 CLINICAL STUDIESTwo clinical studies were conducted to evaluate the efficacy of Clindesse for the treatment of bacterial vaginosis. A clinical diagnosis of bacterial vaginosis was defined by the presence of a ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGClindesse (clindamycin phosphate) Vaginal Cream, 2%, is available in cartons containing one single-dose, pre-filled disposable applicator (NDC 45802-042-01). Each applicator delivers ...
-
17 PATIENT COUNSELING INFORMATIONVaginal Intercourse and Use with Vaginal Products - Instruct the patient not to engage in vaginal intercourse, or use other vaginal products (such as tampons or douches) during treatment with this ...
-
Patient InformationPATIENT INFORMATION - Clindesse (clin-DESS) (clindamycin phosphate) Vaginal Cream, 2% Important Information: Clindesse is for intravaginal use only. Do not use in the eyes, mouth ...
-
Instructions for Use Clindesse (clin-DESS) (clindamycin phosphate) Vaginal Cream, 2% For vaginal use only. Do not put Clindesse in your eyes, mouth, or on your skin. It is important that you read and follow ...
-
Package/Label Display Panel - CartonClindesse® (clindamycin phosphate) Vaginal Cream, 2% NDC 45802-042-01 - Rx Only - This applicator delivers approximately 5 g of vaginal cream containing approximately 100 mg of clindamycin. One ...
-
INGREDIENTS AND APPEARANCEProduct Information