Label: CLINDAMYCIN PHOSPHATE AND TRETINION- clindamycin phosphate and tretinoin gel
- NDC Code(s): 66993-959-31, 66993-959-61
- Packager: Prasco Laboratories
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated August 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Clindamycin Phosphate and Tretinoin Gel safely and - effectively. See full prescribing information for Clindamycin Phosphate ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEClindamycin Phosphate and Tretinoin Gel, 1.2%/0.025% is indicated for the topical treatment of acne vulgaris in - patients 12 years and ...
-
2 DOSAGE AND ADMINISTRATIONClindamycin Phosphate and Tretinoin Gel should be applied once daily in the evening, gently - rubbing the medication to lightly cover the entire affected area ...
-
3 DOSAGE FORMS AND STRENGTHSClindamycin Phosphate and Tretinoin Gel, containing clindamycin phosphate 1.2% and tretinoin - 0.025%, is a yellow, opaque topical gel. Each gram of Clindamycin Phosphate ...
-
4 CONTRAINDICATIONSClindamycin Phosphate and Tretinoin Gel is contraindicated in patients with regional - enteritis, ulcerative colitis, or history of antibiotic-associated ...
-
5 WARNINGS AND PRECAUTIONS5.1 Colitis - Systemic absorption of clindamycin has been demonstrated - following topical use. Diarrhea, bloody diarrhea, and colitis ...
-
6 ADVERSE REACTIONS6.1 Adverse Reactions in Clinical Trials - Because clinical trials are conducted under widely - varying conditions, adverse reaction rates observed in clinical ...
-
7 DRUG INTERACTIONS7.1 Erythromycin - Clindamycin Phosphate and Tretinoin Gel should not be used in combination with - erythromycin-containing products due to possible antagonism ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C. There are no well-controlled studies in pregnant women - treated with Clindamycin Phosphate and Tretinoin Gel. Clindamycin ...
-
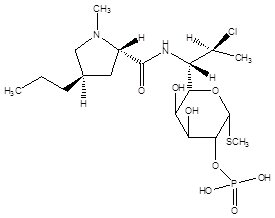
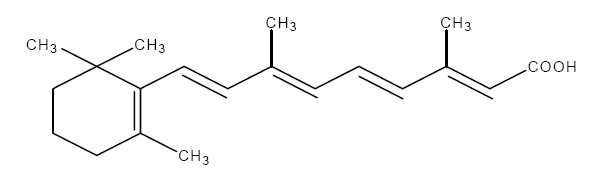
11 DESCRIPTIONClindamycin Phosphate and Tretinoin Gel, 1.2%/0.025%, is - a fixed combination of 2 solubilized active ingredients in an - aqueous-based gel ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Clindamycin:[See Microbiology - (12.4).] Tretinoin: Although the exact mode of action ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to - evaluate the carcinogenic potential of ...
-
14 CLINICAL STUDIESThe safety and efficacy of Clindamycin Phosphate and Tretinoin Gel, applied once daily for the - treatment of acne vulgaris, was evaluated in 12-week multi-center ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Clindamycin Phosphate and Tretinoin Gel is supplied as follows: 30 g aluminum tubes NDC 66993-959-31 - 60 g aluminum tubes NDC 66993-959-61 - Storage and Handling - Store at 25°C ...
-
17 PATIENT COUNSELING INFORMATIONSee FDA-approved patient labeling (Patient - Information). Instructions for Use - At bedtime, the face should be gently ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - Clindamycin Phosphate and Tretinoin Gel, 1.2%/0.025% IMPORTANT: For use on skin only (topical use). Do not get Clindamycin Phosphate ...
-
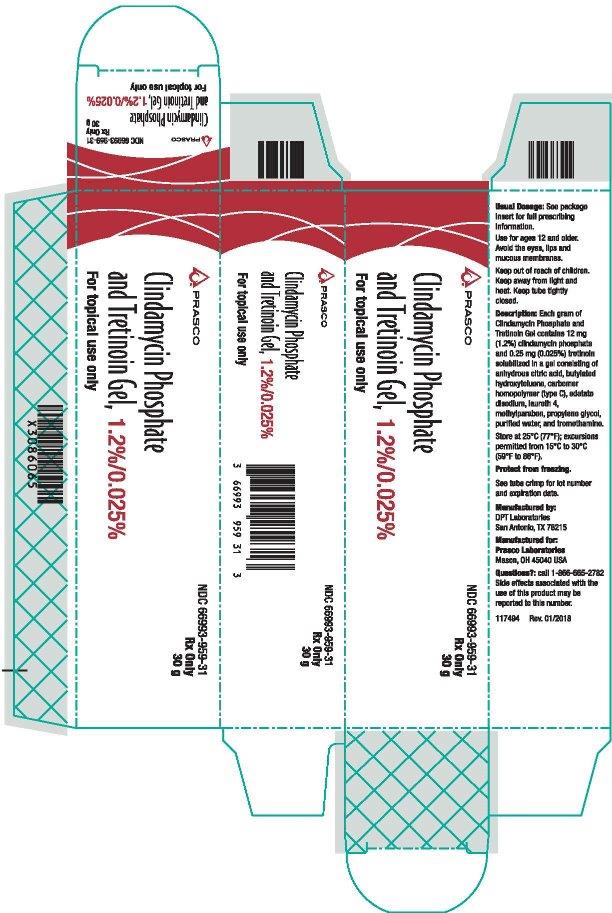
PRINCIPAL DISPLAY PANEL - NDC: 66993-959-31- 30 g Carton Label
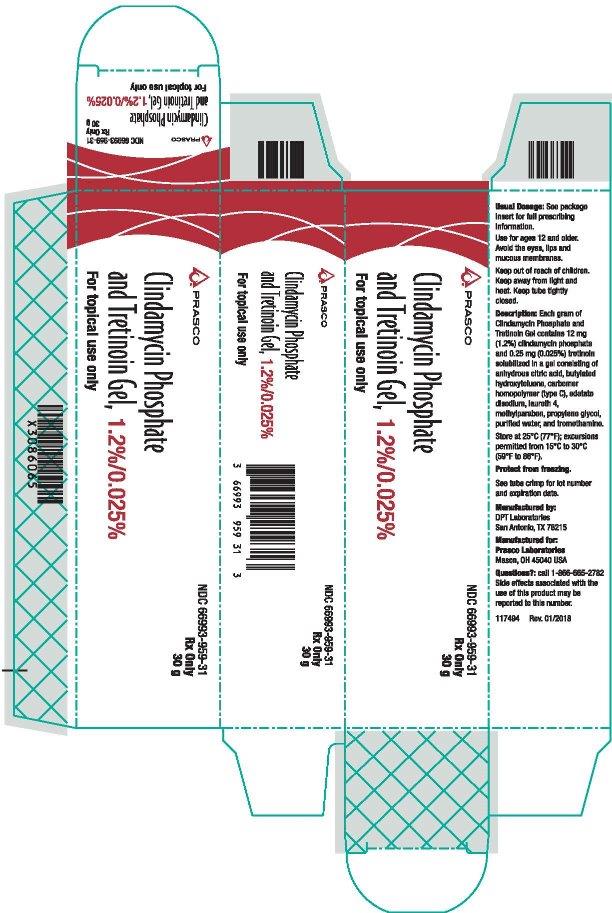
-
PRINCIPAL DISPLAY PANEL - NDC: 66993-959-31 - 30 g Tube Label

-
PRINCIPAL DISPLAY PANEL - NDC: 66993-959-61- 60 g Carton Label

-
PRINCIPAL DISPLAY PANEL - NDC: 66993-959-61 - 60 g Tube Label

-
INGREDIENTS AND APPEARANCEProduct Information