Label: CLENPIQ- sodium picosulfate, magnesium oxide, and anhydrous citric acid liquid
- NDC Code(s): 55566-6800-0, 55566-6800-1
- Packager: Ferring Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 4, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CLENPIQ® safely and effectively. See full prescribing information for CLENPIQ. CLENPIQ® (sodium picosulfate, magnesium oxide, and ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECLENPIQ is indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients 9 years of age and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - Correct fluid and electrolyte abnormalities before administration of CLENPIQ [see Warnings and Precautions (5.1)]. CLENPIQ is ready to drink. It is a ...
-
3 DOSAGE FORMS AND STRENGTHSOral solution: Each bottle contains 10 mg of sodium picosulfate, 3.5 grams of magnesium oxide, and 12 grams of anhydrous citric acid in 175 mL of colorless to slightly yellow, clear solution with ...
-
4 CONTRAINDICATIONSCLENPIQ is contraindicated in the following conditions: Patients with severe renal impairment (creatinine clearance less than 30 mL/minute), which may result in accumulation of magnesium [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Fluid and Electrolyte Abnormalities - Advise patients to hydrate adequately before, during, and after the use of CLENPIQ. Use caution in patients with congestive heart failure when ...
-
6 ADVERSE REACTIONSThe following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling: Serious Fluid and Electrolyte Abnormalities [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Drugs That May Increase Risks of Fluid and Electrolyte Abnormalities - Use caution when prescribing CLENPIQ for patients with conditions or who are taking other drugs, that increase the risk ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data with CLENPIQ use in pregnant women to determine a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, no ...
-
10 OVERDOSAGEOverdosage of more than the recommended dose of CLENPIQ may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances [see ...
-
11 DESCRIPTIONCLENPIQ (sodium picosulfate, magnesium oxide, and anhydrous citric acid) oral solution is a stimulant and osmotic laxative that is provided as a cranberry-flavored, colorless to slightly yellow ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sodium picosulfate is hydrolyzed by colonic bacteria to form an active metabolite: bis-(p-hydroxy-phenyl)-pyridyl-2-methane, BHPM, which acts directly on the colonic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with ...
-
14 CLINICAL STUDIESAdults - Clinical Study with CLENPIQ – Study 1 - The colon-cleansing efficacy of CLENPIQ was evaluated in a randomized, investigator-blinded, active-controlled, multicenter non-inferiority ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - CLENPIQ is supplied in a carton containing two bottles, each holding 175 mL of cranberry-flavored, colorless to slightly yellow, clear oral solution with possible presence of ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use). Instruct patients: CLENPIQ is ready to drink. It is a clear solution with possible ...
-
SPL UNCLASSIFIED SECTIONManufactured by: ASM Aerosol-Service AG - Industriestrasse 11, CH - 4313 Möhlin - Switzerland - Manufactured for: Ferring Pharmaceuticals Inc. Parsippany, N.J. 07054 - Rev. 10/2024
-
MEDICATION GUIDEMEDICATION GUIDE - CLENPIQ® (CLEN-pik) (sodium picosulfate, magnesium oxide, and anhydrous citric acid) oral solution - This Medication Guide has been approved by the U.S. Food and Drug ...
-
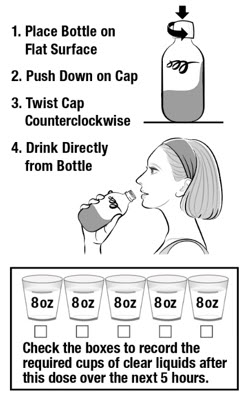
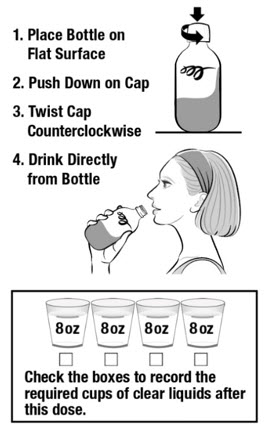
Instructions for UseCLENPIQ® (CLEN-pik)(sodium picosulfate, magnesium oxide, and anhydrous citric acid) oral solutionNOTE: Do not refrigerate or freeze CLENPIQ. CLENPIQ is ready to drink and does not need to be mixed with anything. CLENPIQ is a clear liquid that may have particles. READ BEFORE Taking ...
-
PRINCIPAL DISPLAY PANEL - 175 mL Bottle CartonCranberry - Flavor - NDC 55566-6800-1 - CLENPIQ® (sodium picosulfate, magnesium oxide, and anhydrous citric acid) Oral Solution - 10 mg/3.5 g/12 g per 175 mL bottle - CLENPIQ® is a ready-to-drink - oral ...
-
INGREDIENTS AND APPEARANCEProduct Information