Label: CINVANTI- aprepitant injection, emulsion
- NDC Code(s): 47426-201-01
- Packager: Heron Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CINVANTI® safely and effectively. See full prescribing information for CINVANTI. CINVANTI® (aprepitant) injectable emulsion, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECINVANTI, in combination with other antiemetic agents, is indicated in adults for the prevention of: acute and delayed nausea and vomiting associated with initial and repeat courses of highly ...
-
2 DOSAGE AND ADMINISTRATION2.1 Prevention of Nausea and Vomiting Associated with HEC and MEC - The recommended dosages in adults of CINVANTI, dexamethasone, and a 5-HT3 antagonist for the prevention of nausea and vomiting ...
-
3 DOSAGE FORMS AND STRENGTHSInjectable emulsion: 130 mg/18 mL (7.2 mg/mL) aprepitant as an opaque, off-white to amber emulsion, in single-dose vial
-
4 CONTRAINDICATIONSCINVANTI is contraindicated in patients: who are hypersensitive to any component of the product [see Description (11)]. Hypersensitivity reactions including anaphylaxis have been reported [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Clinically Significant CYP3A4 Drug Interactions - Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Use of CINVANTI with other drugs that are ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Hypersensitivity Reactions [see Warnings and Precautions (5.2)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Effect of Aprepitant on the Pharmacokinetics of Other Drugs - Aprepitant is a substrate, weak-to-moderate (dose-dependent) inhibitor, and an inducer of CYP3A4. Aprepitant is also an inducer ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on CINVANTI use in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Avoid use of CINVANTI in ...
-
10 OVERDOSAGEThere is no specific information on the treatment of overdosage with aprepitant. In the event of overdose, CINVANTI should be discontinued and general supportive treatment and monitoring should be ...
-
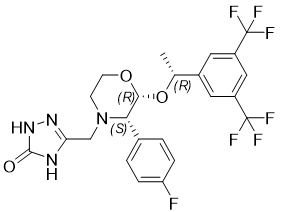
11 DESCRIPTIONCINVANTI injectable emulsion contains the active ingredient, aprepitant. Aprepitant is a substance P/neurokinin 1 (NK1) receptor antagonist, an antiemetic agent, chemically described as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3) ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies were conducted in Sprague-Dawley rats and in CD-1 mice for 2 years. In the rat ...
-
14 CLINICAL STUDIESThe safety and efficacy of CINVANTI have been established based on adequate and well-controlled adult studies of a single-dose of intravenous fosaprepitant, a prodrug of aprepitant, and a 3-day ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCINVANTI injectable emulsion is supplied as an opaque, off-white to amber emulsion in a single-dose glass vial containing 130 mg/18 mL (7.2 mg/mL) aprepitant: NDC 47426-201-011 single-dose ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Hypersensitivity - Advise patients that hypersensitivity reactions, including anaphylaxis, have been reported ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Heron Therapeutics, Inc., San Diego, CA 92121, USA - Patent: https://herontx.com/patents/ CINVANTI® is a registered trademark of Heron Therapeutics, Inc. Copyright © 2017-2024 Heron ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - CINVANTI® (sin van' tee) (aprepitant) injectable emulsion, for intravenous use - This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 130 mg/18 mL Vial CartonNDC 47426-201-01 - Rx Only - CINVANTI ® (aprepitant) injectable emulsion - 130 mg/18 mL - (7.2 mg/mL) For Intravenous Use Only - Must be refrigerated. Store at - 2°C-8°C (36°F-46°F). Do Not Freeze. 1 Sterile ...
-
PRINCIPAL DISPLAY PANEL - 130 mg/18 mL Vial Carton - Not For SaleNDC 47426-201-01 - Rx Only - CINVANTI ® (aprepitant) injectable emulsion - 130 mg/18 mL - (7.2 mg/mL) For Intravenous Use Only - Must be refrigerated. Store at - 2°C-8°C (36°F-46°F). Do Not Freeze. 1 Sterile ...
-
INGREDIENTS AND APPEARANCEProduct Information