Label: CICLODAN- ciclopirox solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 43538-500-06 - Packager: Medimetriks Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 21, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only - For use on fingernails and toenails and immediately adjacent skin only - Not for use in eyes
-
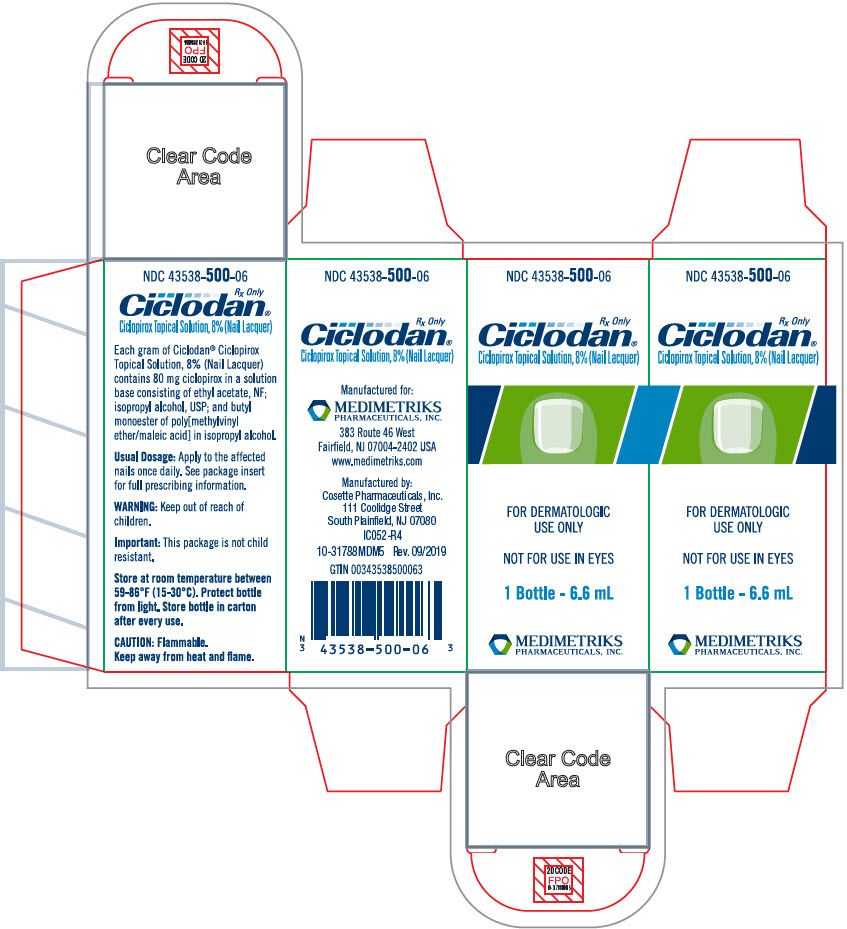
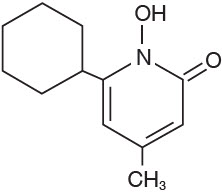
DESCRIPTIONCiclodan® Ciclopirox Topical Solution, 8%, (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent ...
-
CLINICAL PHARMACOLOGYMicrobiology - Mechanism of Action - The mechanism of action of ciclopirox has been investigated using various in vitro and in vivo infection models. One in vitro study suggested that ciclopirox ...
-
INDICATIONS AND USAGE(To understand fully the indication for this product, please read the entire INDICATIONS AND USAGE section of the labeling.) Ciclodan® Ciclopirox Topical Solution, 8%, (Nail Lacquer), as a ...
-
CONTRAINDICATIONSCiclopirox Topical Solution, 8%, (Nail Lacquer), is contraindicated in individuals who have shown hypersensitivity to any of its components.
-
WARNINGSCiclopirox Topical Solution, 8%, (Nail Lacquer), is not for ophthalmic, oral, or intravaginal use. For use on nails and immediately adjacent skin only.
-
PRECAUTIONSIf a reaction suggesting sensitivity or chemical irritation should occur with the use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), treatment should be discontinued and appropriate therapy ...
-
ADVERSE REACTIONSIn the vehicle-controlled clinical trials conducted in the US, 9% (30/327) of patients treated with Ciclopirox Topical Solution, 8%, (Nail Lacquer), and 7% (23/328) of patients treated with ...
-
DOSAGE AND ADMINISTRATIONCiclopirox Topical Solution, 8%, (Nail Lacquer), should be used as a component of a comprehensive management program for onychomycosis. Removal of the unattached, infected nail, as frequently as ...
-
HOW SUPPLIEDCiclodan® Ciclopirox Topical Solution, 8%, (Nail Lacquer), is supplied in a 6.6 mL (NDC 43538-500-06) glass bottle with a screw cap which is fitted with a brush. Protect from light (e.g. ...
-
ReferencesDittmar W., Lohaus G. 1973. HOE296, A new antimycotic compound with a broad antimicrobial spectrum. Arzneim-Forsch./Drug Res. 23:670-674. Niewerth et. al., 1998. Antimicrobial susceptibility ...
-
SPL UNCLASSIFIED SECTIONGantrez is a registered trademark of GAF Corporation - You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call ...
-
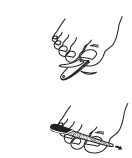
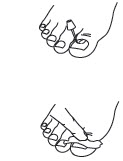
PATIENT PACKAGE INSERTCiclodan® Ciclopirox - Topical Solution, 8% (Nail Lacquer) Rx Only - Ciclopirox Topical Solution, 8%, (Nail Lacquer) Patient Information and Instructions - Patients should have detailed ...
-
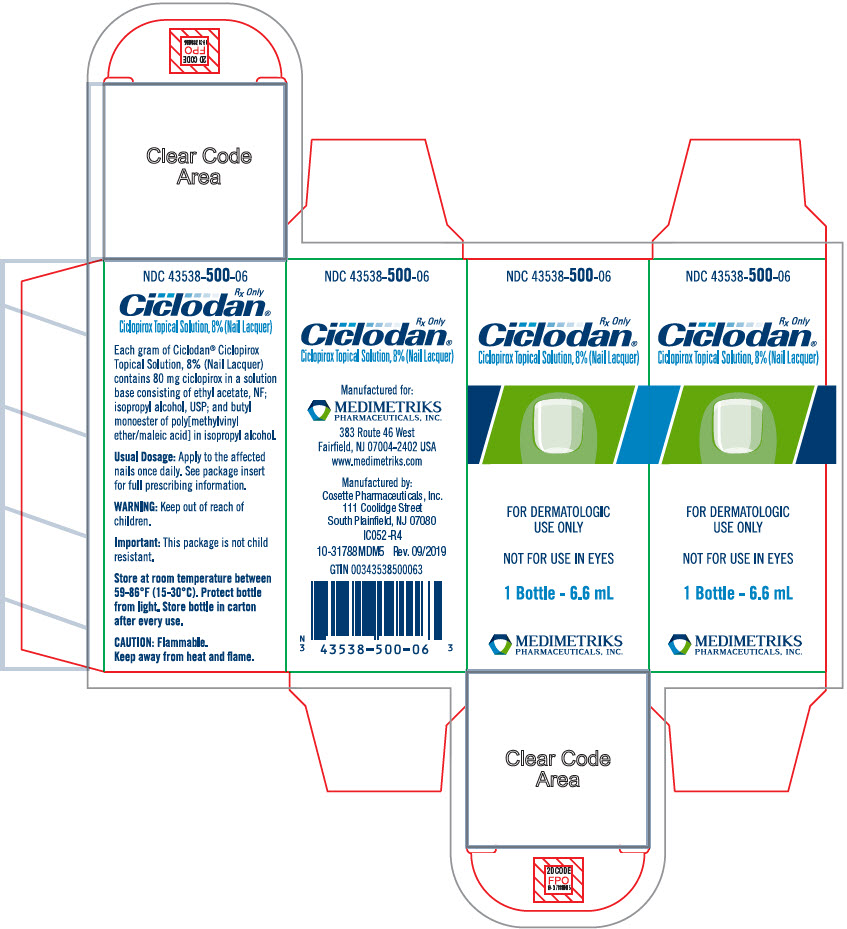
PRINCIPAL DISPLAY PANEL - 6.6 mL Bottle CartonNDC 43538-500-06 - Rx Only - Ciclodan® Ciclopirox Topical Solution, 8% (Nail Lacquer) FOR DERMATOLOGIC - USE ONLY - NOT FOR USE IN EYES - 1 Bottle - 6.6 mL - MEDIMETRIKS - PHARMACEUTICALS, INC.
-
INGREDIENTS AND APPEARANCEProduct Information