Label: CETROTIDE- cetrorelix acetate kit
- NDC Code(s): 44087-1225-1
- Packager: EMD Serono, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR SUBCUTANEOUS USE ONLY
-
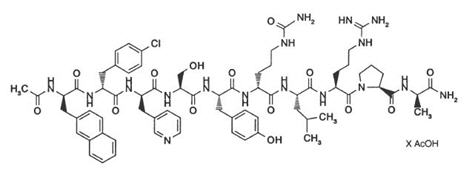
DESCRIPTIONCetrotide® (cetrorelix acetate for injection) is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH) antagonistic activity. Cetrorelix acetate is an analog of native GnRH with ...
-
CLINICAL PHARMACOLOGYGnRH induces the production and release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the gonadotrophic cells of the anterior pituitary. Due to a positive estradiol (E2 ...
-
INDICATIONS AND USAGECetrotide® (cetrorelix acetate for injection) is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian stimulation.
-
CONTRAINDICATIONSCetrotide® (cetrorelix acetate for injection) is contraindicated under the following conditions: Hypersensitivity to cetrorelix acetate, extrinsic peptide hormones or mannitol. Known ...
-
WARNINGSCetrotide® (cetrorelix acetate for injection) should be prescribed by physicians who are experienced in fertility treatment. Before starting treatment with Cetrotide®, pregnancy must be excluded ...
-
PRECAUTIONSGeneral - Cases of hypersensitivity reactions, including anaphylactoid reactions with the first dose, have been reported during post-marketing surveillance (see ADVERSE REACTIONS). A severe ...
-
ADVERSE REACTIONSThe safety of Cetrotide® (cetrorelix acetate for injection) in 949 patients undergoing controlled ovarian stimulation in clinical studies was evaluated. Women were between 19 and 40 years of age ...
-
OVERDOSAGEThere have been no reports of overdosage with Cetrotide® 0.25 mg or 3 mg in humans. Single doses up to 120 mg Cetrotide® have been well tolerated in patients treated for other indications without ...
-
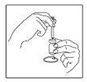
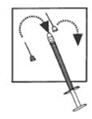
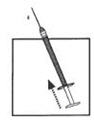
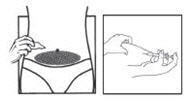
DOSAGE AND ADMINISTRATIONOvarian stimulation therapy with gonadotropins (FSH, hMG) is started on cycle Day 2 or 3. The dose of gonadotropins should be adjusted according to individual response. Cetrotide® (cetrorelix ...
-
HOW SUPPLIEDCetrotide® (cetrorelix acetate for injection) 0.25 mg is available in a carton of one packaged tray (NDC 44087-1225-1). Each packaged tray contains: one glass vial containing 0.26 - 0.27 mg ...
-
SPL UNCLASSIFIED SECTIONRx only - Manufactured for: EMD Serono, Inc, Rockland, MA 02370, USA - June 2024
-
Patient LeafletCetrotide® 0.25 mg - Active ingredient: cetrorelix acetate - Summary - Cetrotide® blocks the effects of a natural hormone, called gonadotropin-releasing hormone (GnRH). GnRH controls the secretion ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonCetrotide® 0.25 mg - (cetrorelix acetate for injection) NDC 44087-1225-1 - Sterile - for subcutaneous use only - Rx only - Store refrigerated, 2-8°C (36-46°F). One carton contains one packaged tray which ...
-
INGREDIENTS AND APPEARANCEProduct Information