Label: CEQUA- cyclosporine solution/ drops
- NDC Code(s): 47335-506-96, 47335-507-97
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 6, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CEQUA safely and effectively. See full prescribing information for CEQUA. CEQUA® (cyclosporine ophthalmic solution) 0.09%, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE CEQUA ophthalmic solution is a calcineurin inhibitor immunosuppressant indicated to increase tear production in patients with keratoconjunctivitis sicca (dry eye). (1)
-
2 DOSAGE AND ADMINISTRATION Instill one drop of CEQUA twice daily (approximately 12 hours apart) into each eye. CEQUA can be used concomitantly with artificial tears, allowing a 15 minute interval between products. Discard ...
-
3 DOSAGE FORMS AND STRENGTHS Ophthalmic solution containing cyclosporine 0.9 mg/mL (3)
-
4 CONTRAINDICATIONS None. (4)
-
5 WARNINGS AND PRECAUTIONS 5.1 Potential for Eye Injury and Contamination - To avoid the potential for eye injury and contamination, advise patients not to touch the vial tip to the eye or other surfaces. 5.2 Use with ...
-
6 ADVERSE REACTIONS 6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of CEQUA administration in pregnant women to inform a drug-associated risk. Oral administration of cyclosporine ...
-
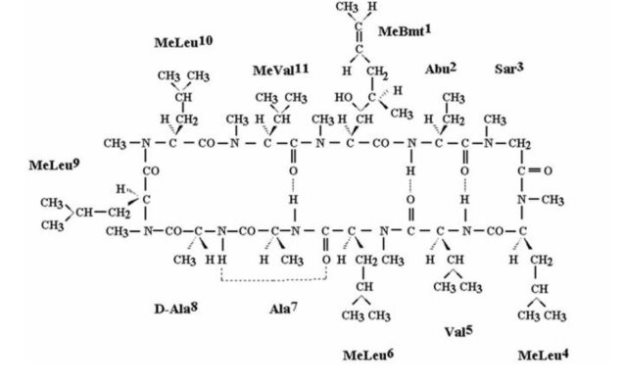
11 DESCRIPTION CEQUA (cyclosporine ophthalmic solution) 0.09% contains a topical calcineurin inhibitor immunosuppressant. Cyclosporine’s chemical name is ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Cyclosporine is a calcineurin inhibitor immunosuppressant agent when administered systemically. In patients whose tear production is presumed to be suppressed due to ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Systemic carcinogenicity studies were carried out in male and female mice and rats. In the 78-week oral (diet) mouse ...
-
14 CLINICAL STUDIES Two multicenter, randomized, adequate and well-controlled clinical studies treated 1,048 patients with keratoconjunctivitis sicca (NCT # 02254265 and NCT # 02688556). In both studies, compared to ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING CEQUA ophthalmic solution is packaged in sterile, preservative-free, single-use vials. Each vial - contains 0.25 mL fill in a 0.9 mL LDPE vial; 10 vials (2 cards of 5 vials) are packaged in ...
-
17 PATIENT COUNSELING INFORMATION Handling the Vial - Advise patients to not allow the tip of the vial to touch the eye or any surface, as this may - contaminate the solution. Advise patients also not to touch the vial tip to their ...
-
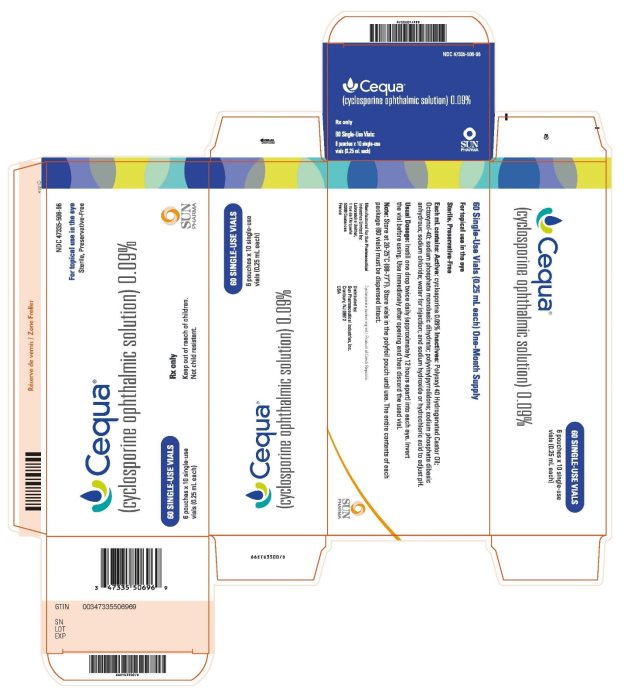
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 47355-506-96 - For topical use in the eye - sterile, Preservative-Free - Cequa® (cyclosporine ophthalmic solution) 0.09% 60 SINGLE-USE VIALS - 6 pouches x 10 ...
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 47355-507-97 - For topical use in the eye - sterile, Preservative-Free - Cequa® (cyclosporine ophthalmic solution) 0.09% 10 SINGLE-USE SAMPLE VIALS - 1 pouches x ...
-
INGREDIENTS AND APPEARANCEProduct Information