Label: INQOVI- cedazuridine and decitabine tablet, film coated
- NDC Code(s): 64842-0727-9
- Packager: Taiho Pharmaceutical Co., Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use INQOVI safely and effectively. See full prescribing information for INQOVI. INQOVI® (decitabine and cedazuridine) tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEINQOVI is indicated for treatment of adult patients with myelodysplastic syndromes (MDS), including previously treated and untreated, de novo and secondary MDS with the following ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Information - Do NOT substitute INQOVI for an intravenous decitabine product within a cycle. Consider administering antiemetics prior to each dose to minimize nausea ...
-
3 DOSAGE FORMS AND STRENGTHSINQOVI tablets contain 35 mg decitabine and 100 mg cedazuridine. The tablets are biconvex, oval-shaped, film-coated, red and debossed with “H35” on one side.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Myelosuppression - Fatal and serious myelosuppression can occur with INQOVI. Based on laboratory values, new or worsening thrombocytopenia occurred in 82% of patients, with Grade 3 or 4 ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because ...
-
7 DRUG INTERACTIONS7.1 Effects of INQOVI on Other Drugs - Drugs Metabolized by Cytidine Deaminase - Cedazuridine is an inhibitor of the cytidine deaminase (CDA) enzyme. Coadministration of INQOVI with drugs that ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on findings from human data, animal studies, and its mechanism of action [see Clinical Pharmacology (12.1)], INQOVI can cause fetal harm when administered to ...
-
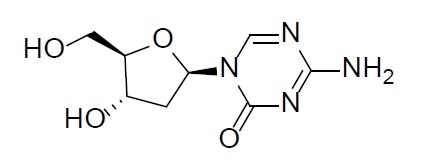
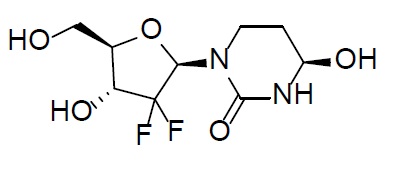
11 DESCRIPTIONDecitabine - Decitabine is a nucleoside metabolic inhibitor. Decitabine is a white to off-white solid with the molecular formula of C8H12N4O4 and a molecular weight of 228.21 daltons. Its ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Decitabine is a nucleoside metabolic inhibitor that is believed to exert its effects after phosphorylation and direct incorporation into DNA and inhibition of DNA ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies with decitabine, cedazuridine, or their combination have not been ...
-
14 CLINICAL STUDIESStudy ASTX727-01-B - INQOVI was evaluated in Study ASTX727-01-B, an open-label, randomized, 2-cycle, 2-sequence crossover study (NCT02103478) that included 80 adult patients with MDS ...
-
15 REFERENCES1. OSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - INQOVI tablets are biconvex, oval-shaped, film-coated, red, and debossed with “H35” on one side. The tablets are packaged in blisters and supplied as follows: NDC: 64842-0727-9; 5 ...
-
STORAGE AND HANDLINGStorage and Handling - Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense medication in the original ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Myelosuppression - Advise patients of the risk of myelosuppression and to report any symptoms of fever ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Taiho Pharmaceutical Co., Ltd - Japan - Distributed by: Taiho Oncology, Inc. Princeton, NJ 08540 USA
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - INQOVI® (IN KOE' VEE) (decitabine and cedazuridine) tablets - This Patient Information has been approved by the U.S. Food and Drug AdministrationIssued ...
-
PRINCIPAL DISPLAY PANEL - 35 mg/100 mg Tablet CartonNDC 64842-0727-9 - Rx Only - INQOVI® (decitabine and cedazuridine) tablets - 35 mg/100 mg per tablet - CAUTION: Hazardous Agent - One Blister card - containing 5 tablets - TAIHO - ONCOLOGY ...
-
INGREDIENTS AND APPEARANCEProduct Information