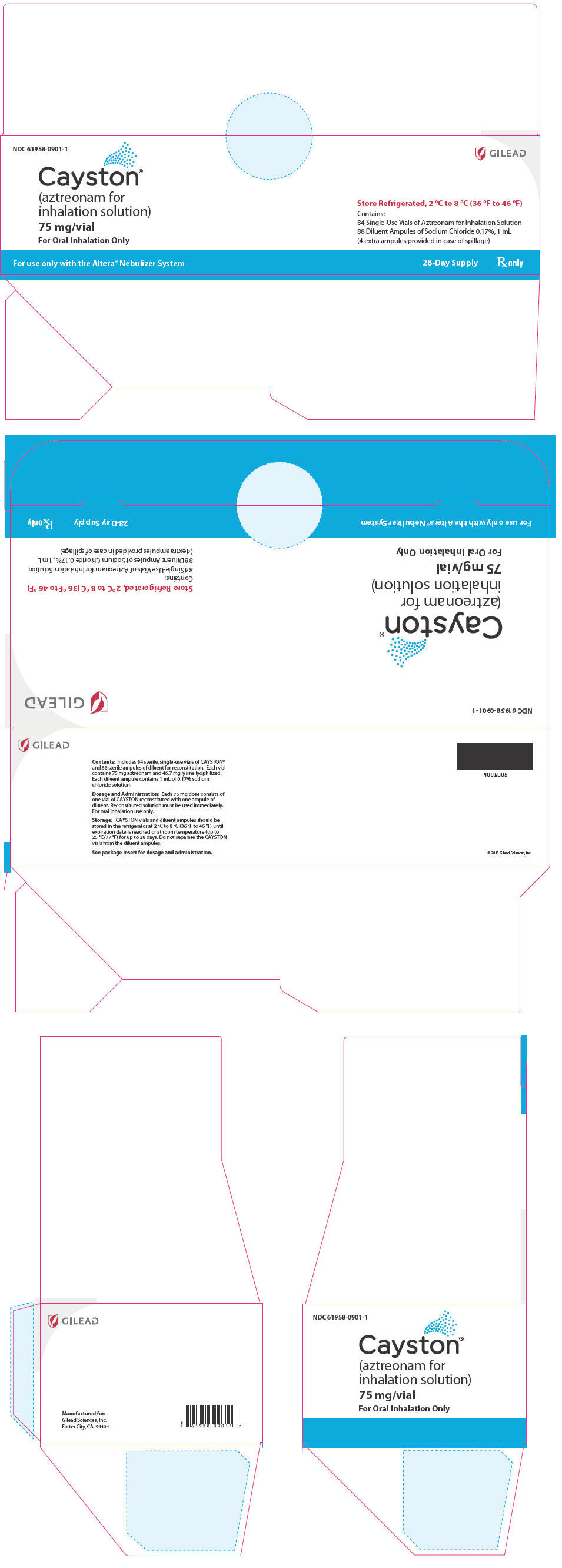
Label: CAYSTON- aztreonam kit
- NDC Code(s): 61958-0901-1
- Packager: Gilead Sciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CAYSTON safely and effectively. See full prescribing information for CAYSTON. CAYSTON® (aztreonam for inhalation solution), for ...
-
Table of ContentsTable of Contents
- MICROBIOLOGY
-
1 INDICATIONS AND USAGECAYSTON® is indicated to improve respiratory symptoms in cystic fibrosis (CF) patients with Pseudomonas aeruginosa. Safety and effectiveness have not been established in pediatric patients below ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The recommended dose of CAYSTON for both adults and pediatric patients 7 years of age and older is one single-use vial (75 mg of aztreonam) reconstituted with 1 mL of ...
-
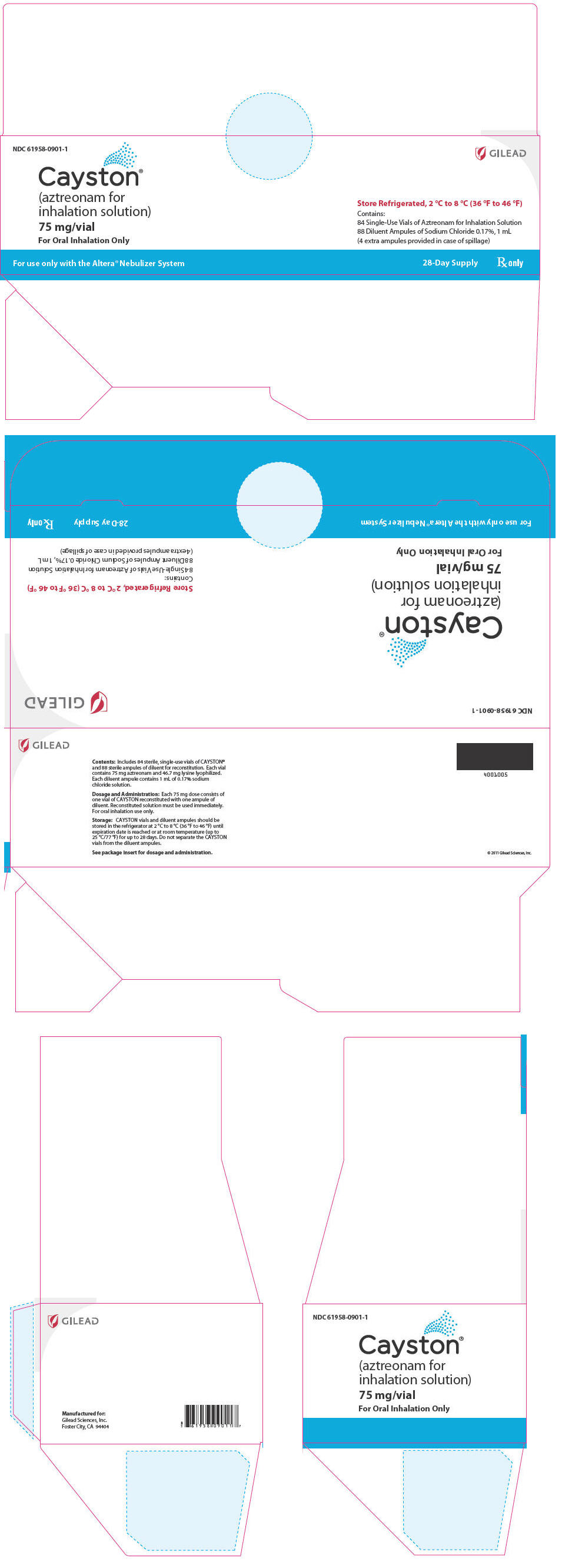
3 DOSAGE FORMS AND STRENGTHSA dose of CAYSTON consists of a single-use vial of sterile, lyophilized aztreonam (75 mg) reconstituted with a 1 mL ampule of sterile diluent (0.17% sodium chloride). Reconstituted CAYSTON is ...
-
4 CONTRAINDICATIONSCAYSTON is contraindicated in patients with a known allergy to aztreonam.
-
5 WARNINGS AND PRECAUTIONS5.1 Allergic Reactions - Severe allergic reactions have been reported following administration of aztreonam for injection to patients with no known history of exposure to aztreonam. In addition ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of drugs cannot be directly compared ...
-
7 DRUG INTERACTIONSNo formal clinical studies of drug interactions with CAYSTON have been conducted.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data on CAYSTON use in pregnant women is insufficient to inform a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
10 OVERDOSAGENo overdoses have been reported with CAYSTON in clinical trials to date. In clinical trials, 225 mg doses of CAYSTON via inhalation were associated with higher rates of drug-related respiratory ...
-
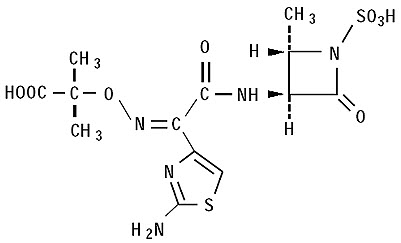
11 DESCRIPTIONA dose of CAYSTON consists of a 2 mL amber glass vial containing lyophilized aztreonam (75 mg) and lysine (46.7 mg), and a low-density polyethylene ampule containing 1 mL sterile diluent (0.17 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Aztreonam is an antibacterial drug [see Clinical Pharmacology (12.4)]. 12.3 Pharmacokinetics - Sputum Concentrations - Sputum aztreonam concentrations exhibited ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - A 104-week rat inhalation toxicology study to assess the carcinogenic potential of aztreonam demonstrated no drug-related increase in ...
-
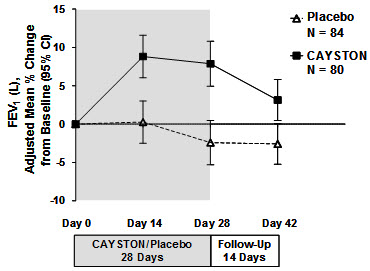
14 CLINICAL STUDIESCAYSTON was evaluated over a period of 28 days of treatment in a randomized, double-blind, placebo-controlled, multicenter trial that enrolled patients with CF and P. aeruginosa. This trial was ...
-
15 REFERENCESClinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically—Eighth Edition; Approved Standard. CLSI Document ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach kit for a 28-day course of CAYSTON contains 84 sterile vials of CAYSTON and 88 ampules of sterile diluent packed in 2 cartons, each carton containing a 14-day supply. The four additional ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Allergic Reactions - Advise patients to tell their healthcare provider immediately ...
-
SPL UNCLASSIFIED SECTIONCAYSTON is a trademark of Gilead Sciences, Inc. All other trademarks referenced herein are the property of their respective owners. © 2019 Gilead Sciences, Inc. All rights ...
-
PATIENT PACKAGE INSERTThis Patient Information has been approved by the U.S. Food and Drug AdministrationRev November 2019 - PATIENT INFORMATION - CAYSTON® (kay-stun) (aztreonam for inhalation solution) for oral ...
-
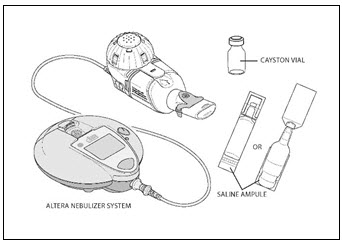
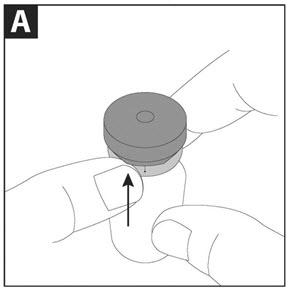
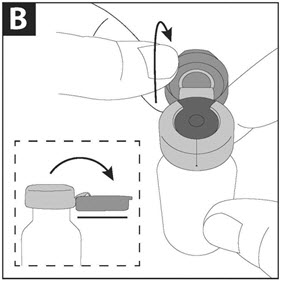
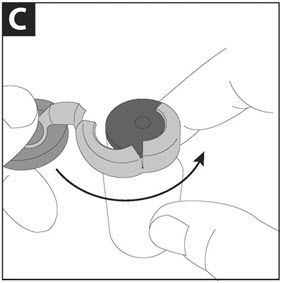
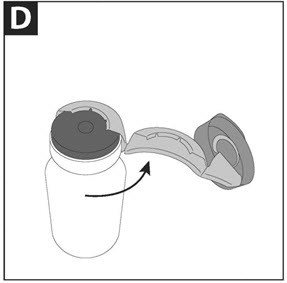
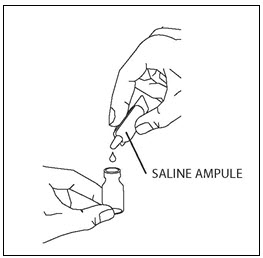
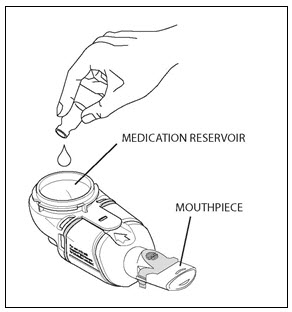
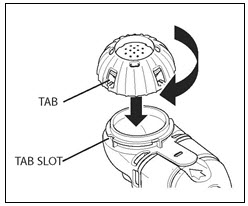
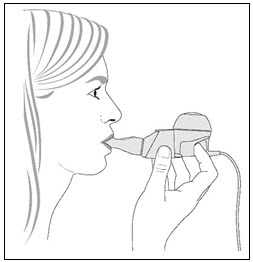
PATIENT INSTRUCTIONS FOR USECAYSTON® (aztreonam for inhalation solution) for oral inhalation use - Be sure that you read, understand and follow the Patient Instructions for Use below for the right way to take CAYSTON. If ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Gilead Sciences, Inc., Foster City, CA 94404 - CAYSTON is a trademark of Gilead Sciences, Inc. All other trademarks referenced herein are the property of their respective owners. ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - Cayston 28 Day Carton - Representative Label - NDC 61958-0901-1 - GILEAD - Cayston® (aztreonam for - inhalation solution) 75 mg/vial - For Oral Inhalation Only - Store ...
-
INGREDIENTS AND APPEARANCEProduct Information