Label: CAPRELSA- vandetanib tablet, film coated
- NDC Code(s): 58468-7820-3, 58468-7840-3
- Packager: Genzyme Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CAPRELSA safely and effectively. See full prescribing information for CAPRELSA. CAPRELSA® (vandetanib) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: QT PROLONGATION, TORSADES DE POINTES, AND SUDDEN DEATH
CAPRELSA can prolong the QT interval. Torsades de pointes and sudden death have occurred in patients receiving CAPRELSA. Do not use CAPRELSA in patients with hypocalcemia, hypokalemia, hypomagnesemia, or long QT syndrome. Correct hypocalcemia, hypokalemia and/or hypomagnesemia prior to CAPRELSA administration. Monitor electrolytes periodically. Avoid drugs known to prolong the QT interval. Only prescribers and pharmacies certified with the restricted distribution program are able to prescribe and dispense CAPRELSA [see Warnings and Precautions (5.1, 5.16)].
Close -
1 INDICATIONS AND USAGECAPRELSA is indicated for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease. Use CAPRELSA in patients with ...
-
2 DOSAGE AND ADMINISTRATIONThe recommended dose of CAPRELSA is 300 mg taken orally once daily until disease progression or unacceptable toxicity occurs. CAPRELSA may be taken with or without food. Do not take a missed dose ...
-
3 DOSAGE FORMS AND STRENGTHSCAPRELSA 100 mg tablets are white, round, biconvex, film-coated, and intagliated with 'Z 100' on one side and plain on the reverse side. CAPRELSA 300 mg tablets are white, oval, biconvex ...
-
4 CONTRAINDICATIONSDo not use in patients with congenital long QT syndrome [see Boxed Warning].
-
5 WARNINGS AND PRECAUTIONS5.1 QT Prolongation and Torsades de Pointes - CAPRELSA can prolong the QT interval in a concentration-dependent manner [see Clinical Pharmacology (12.2)]. Torsades de pointes, ventricular ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are discussed elsewhere in the label: QT Prolongation and Torsades de Pointes [see Boxed Warning, Warnings and Precautions (5.1)] Severe Skin Reactions ...
-
7 DRUG INTERACTIONS7.1 Effect of CYP3A4 Inducers on CAPRELSA - Rifampicin, a strong CYP3A4 inducer, decreased vandetanib plasma concentrations. Avoid concomitant use of known strong CYP3A4 inducers during CAPRELSA ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action and findings in animals, CAPRELSA can cause fetal harm when administered to a pregnant woman. There are no available human data ...
-
10 OVERDOSAGEIn the event of an overdose, monitor patients closely for QTc prolongation. Adverse events including QT interval prolongation should be monitored closely as they may not resolve fully until ...
-
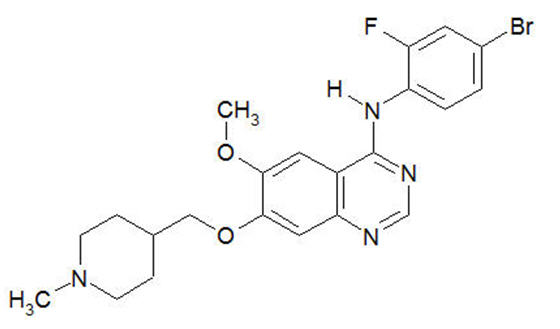
11 DESCRIPTIONVandetanib has the chemical name N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl) methoxy]quinazolin-4-amine. The structural and molecular formulas are: C22H24BrFN4O2 - Vandetanib ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - In vitro studies have shown that vandetanib inhibits the tyrosine kinase activity of the EGFR and VEGFR families, RET, BRK, TIE2, and members of the EPH receptor and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Vandetanib was not carcinogenic in a 2-year study in rats when administered by daily oral gavage at doses of up to 10 mg/kg (0.7 times ...
-
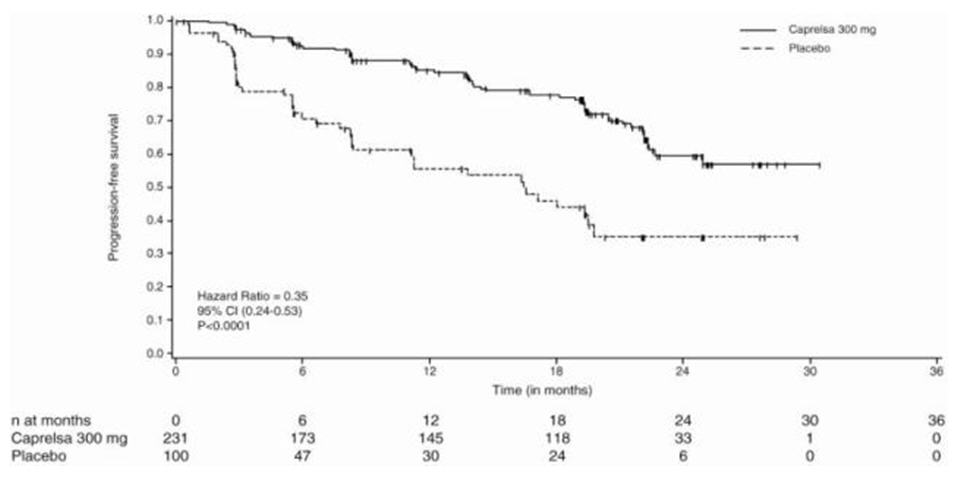
14 CLINICAL STUDIES14.1 Clinical Trial in Patients with Medullary Thyroid Cancer - A double-blind, placebo-controlled study (Study D4200C00058, NCT00410761) randomized patients with unresectable locally advanced ...
-
15 REFERENCESOSHA Hazardous Drugs (OSHA Technical Manual). OSHA.
-
16 HOW SUPPLIED/STORAGE AND HANDLING 100 mg Tablets available in bottles containing 30 tablets (NDC 58468-7820-3). 300 mg Tablets available in bottles containing 30 tablets (NDC 58468-7840-3). 16.1 Storage and Handling - CAPRELSA ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). QT Prolongation and Torsades de Pointes - Advise patients to contact their healthcare provider in the event of ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Genzyme Corporation - Cambridge, MA 02141 - A SANOFI COMPANY - CAPRELSA is a registered trademark of Genzyme Corporation. ©2024 Genzyme Corporation. For patent information ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: April 2024 - MEDICATION GUIDE - CAPRELSA® (kap-rel-sah) (vandetanib) tablets - Read this Medication ...
-
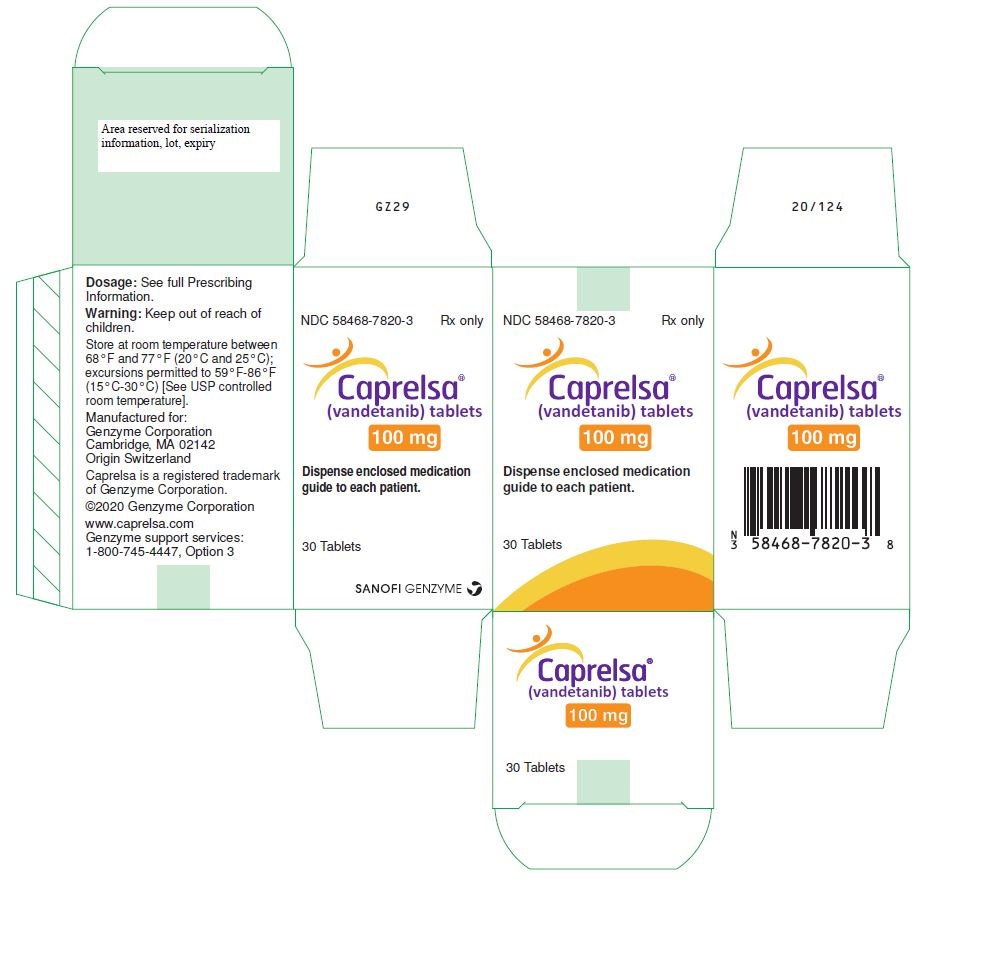
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle CartonNDC 58468-7820-3 Rx only - Caprelsa® (vandetanib) tablets - 100 mg - Dispense enclosed medication - guide to each patient. 30 Tablets
-
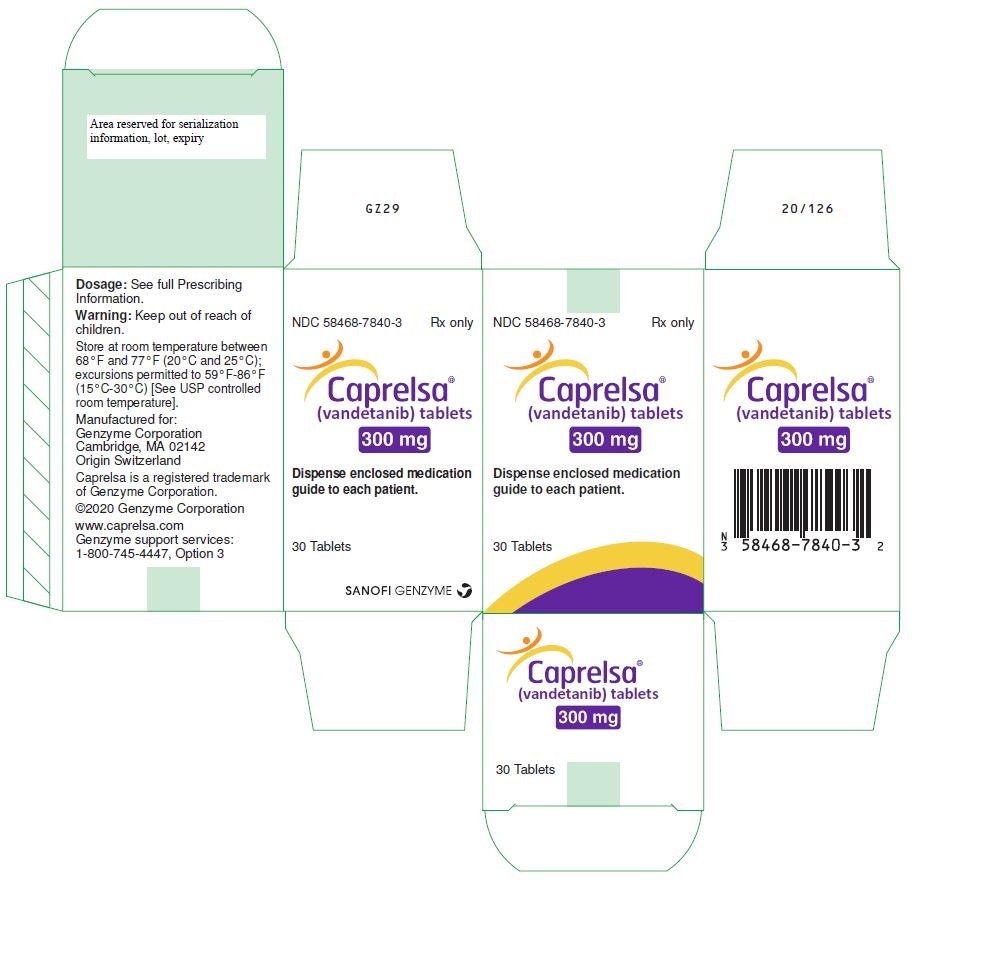
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle CartonNDC 58468-7840-3 Rx only - Caprelsa® (vandetanib) tablets - 300 mg - Dispense enclosed medication - guide to each patient. 30 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information