Label: CABLIVI- caplacizumab kit
- NDC Code(s): 58468-0225-1, 58468-0227-1, 58468-0229-1
- Packager: Genzyme Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CABLIVI® safely and effectively. See full prescribing information for CABLIVI. CABLIVI (caplacizumab-yhdp) for injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGECABLIVI is indicated for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura (aTTP), in combination with plasma exchange and immunosuppressive therapy.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dose and Schedules - CABLIVI should be administered upon initiation of plasma exchange therapy. The recommended dose of CABLIVI is as follows: First day of treatment: 11 mg ...
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 11 mg as a white lyophilized powder in a single-dose vial.
-
4 CONTRAINDICATIONSCABLIVI is contraindicated in patients with a previous severe hypersensitivity reaction to caplacizumab-yhdp or to any of the excipients. Hypersensitivity reactions have included urticaria [see ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hemorrhage - CABLIVI increases the risk of bleeding [see Adverse Reactions (6.1)]. In clinical studies, severe bleeding adverse reactions of epistaxis, gingival bleeding, upper ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are also discussed in other sections of the labeling: Hemorrhage [see Warnings and Precautions (5.1)] 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONSConcomitant Use of Anticoagulants or Antiplatelet Agents - Concomitant use of CABLIVI with any anticoagulant or antiplatelet agent may increase the risk of bleeding. Avoid concomitant use when ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on CABLIVI use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. However, there are ...
-
10 OVERDOSAGEIn case of overdose, based on the pharmacological action of CABLIVI, there is the potential for an increased risk of bleeding [see Warnings and Precautions (5.1)]. Close monitoring for signs and ...
-
11 DESCRIPTIONCaplacizumab-yhdp is a von Willebrand factor (vWF)-directed antibody fragment that consists of two identical humanized building blocks, linked by a three-alanine linker. Caplacizumab-yhdp is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Caplacizumab-yhdp targets the A1-domain of vWF, and inhibits the interaction between vWF and platelets, thereby reducing both vWF-mediated platelet adhesion and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been performed to evaluate the potential of caplacizumab-yhdp for carcinogenicity or genotoxicity. Animal reproduction ...
-
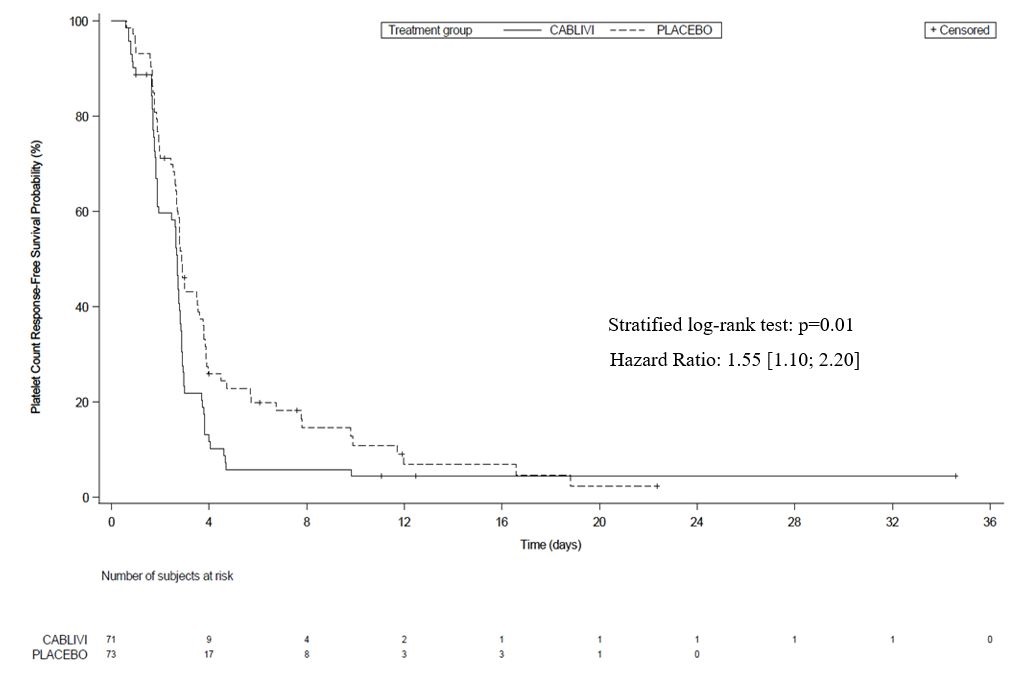
14 CLINICAL STUDIESThe efficacy of CABLIVI for the treatment of adult patients with acquired thrombotic thrombocytopenic purpura (aTTP) in combination with plasma exchange and immunosuppressive therapy was ...
-
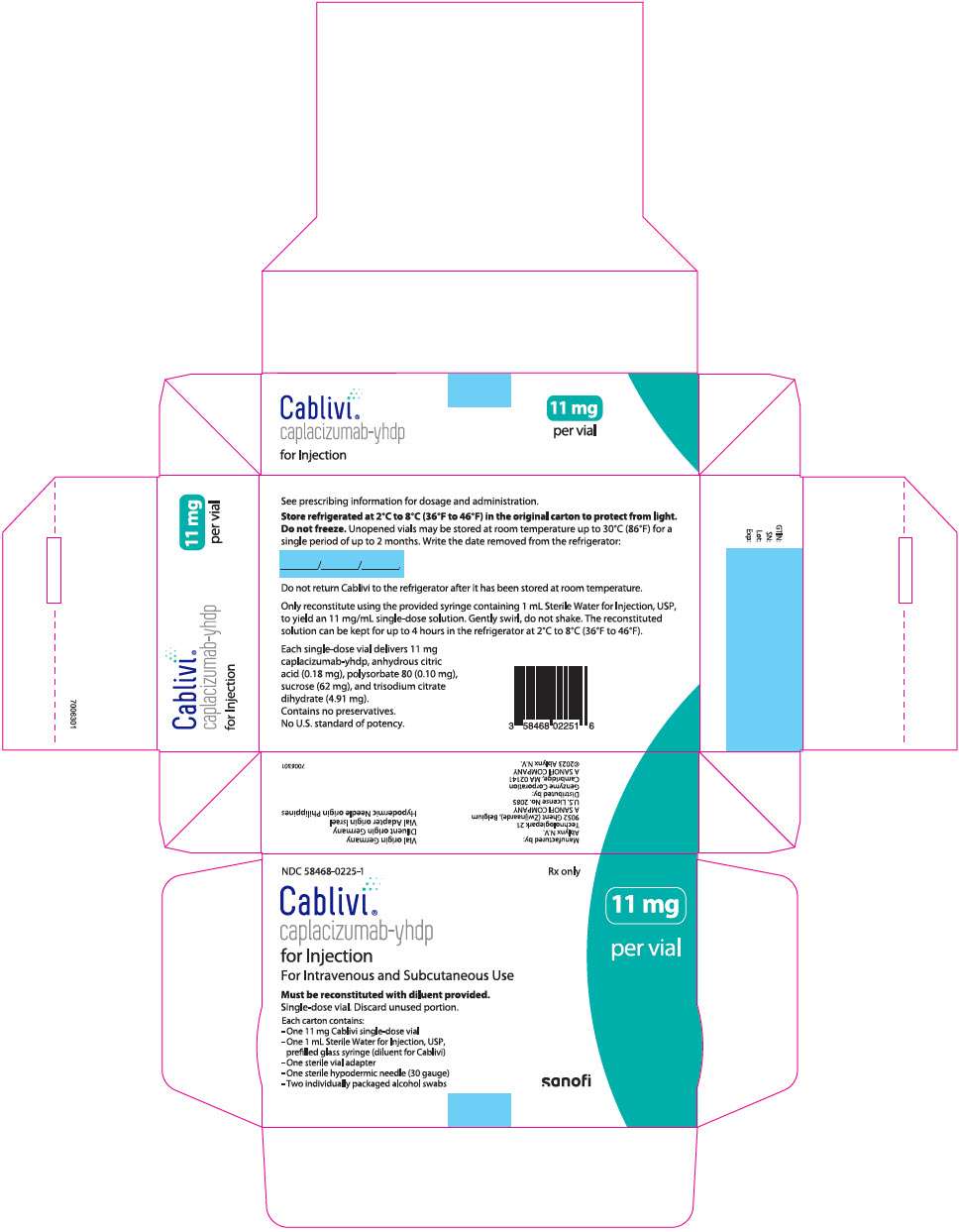
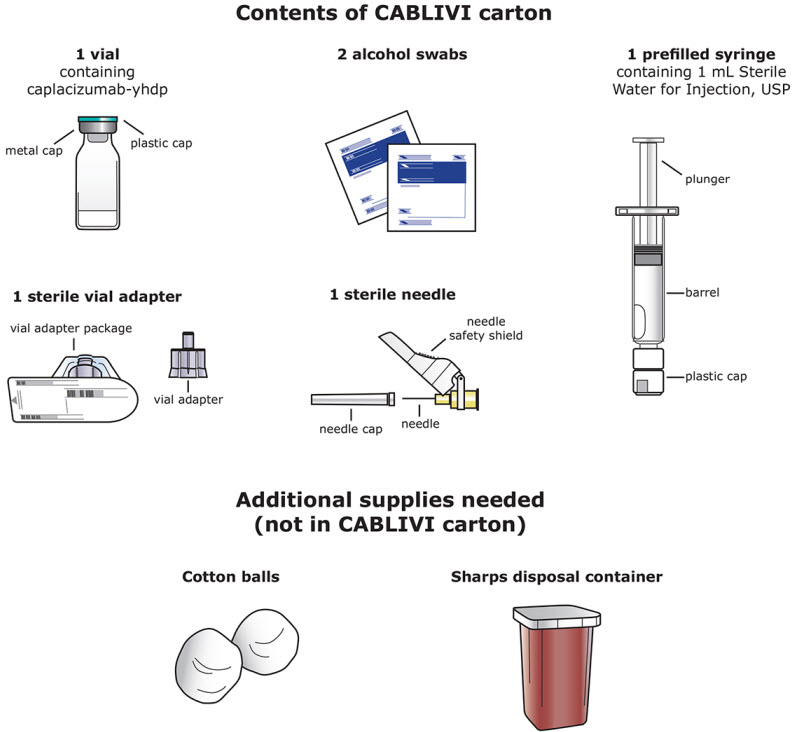
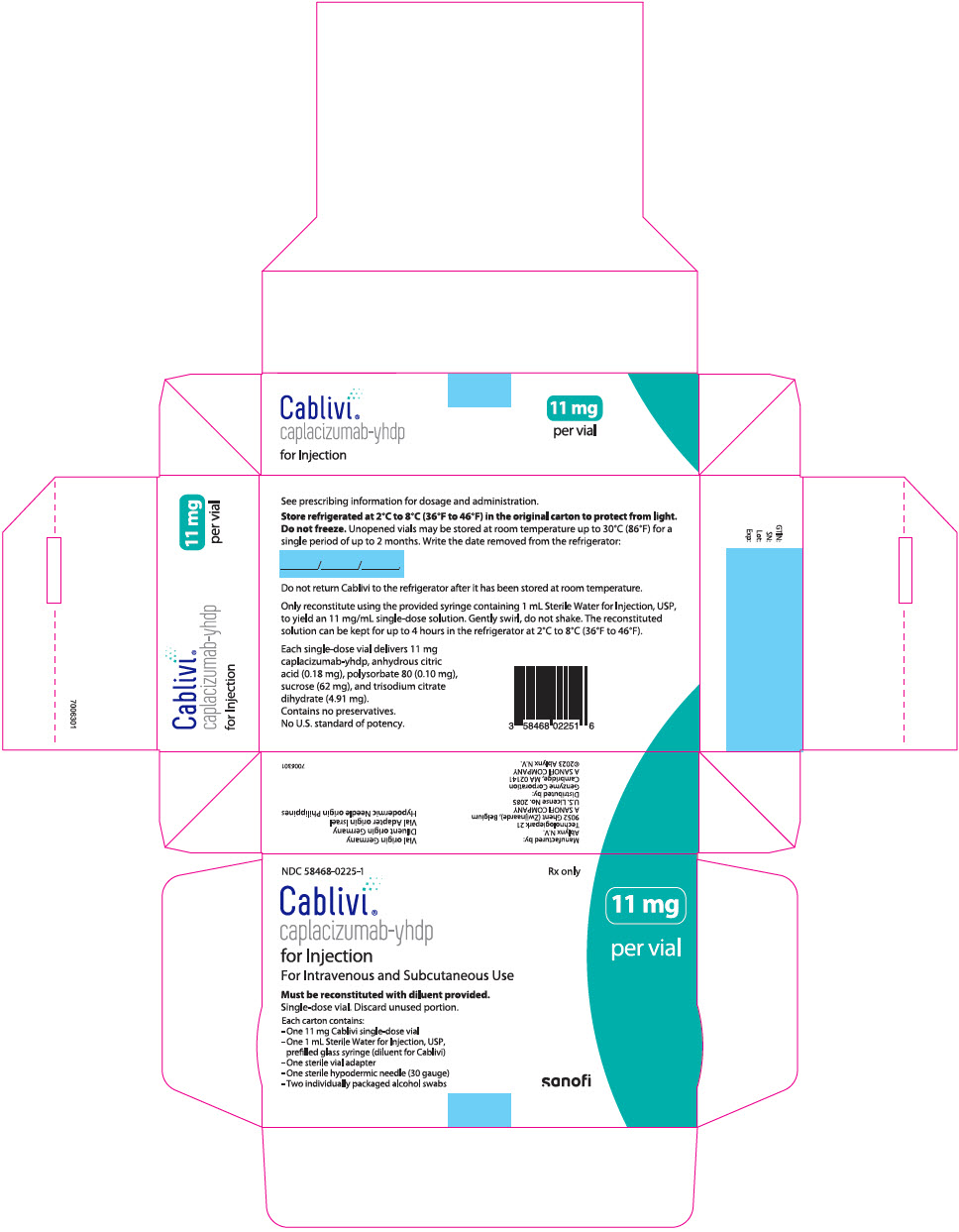
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - CABLIVI (caplacizumab-yhdp) for injection is a sterile, white, preservative-free, lyophilized powder in a single-dose vial. Each carton (NDC 58468-0225-1) contains: one 11 mg ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Bleeding [see Warnings and Precautions (5.1)] Advise patients that bruising ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Ablynx N.V. Technologiepark 21 - 9052 Ghent (Zwijnaarde), Belgium - A SANOFI COMPANY - U.S. License No. 2085 - Distributed by: Genzyme Corporation - Cambridge, MA 02141 - A SANOFI COMPANY - For ...
-
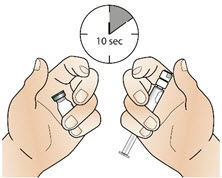
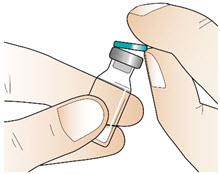
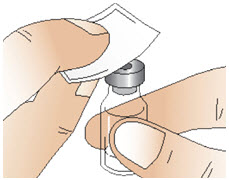
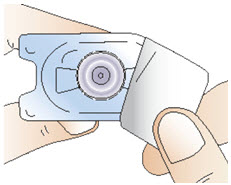
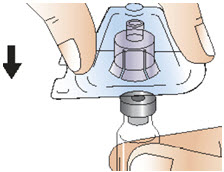
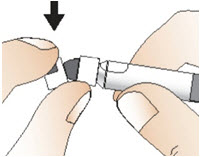
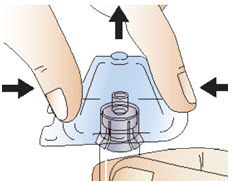
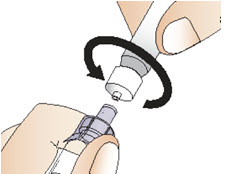
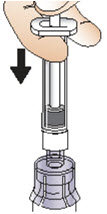
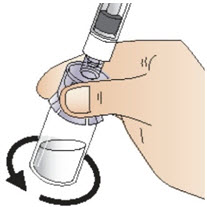
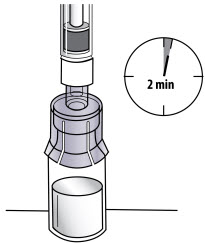
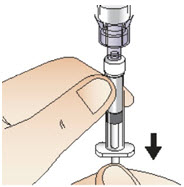
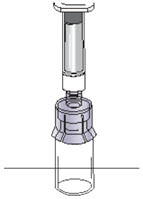
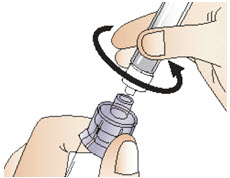
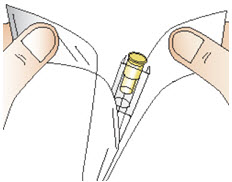
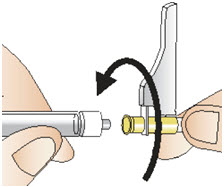
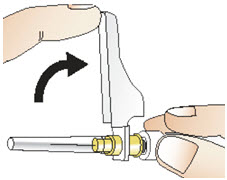
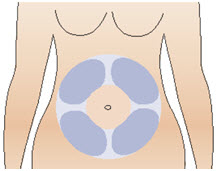
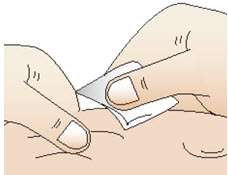
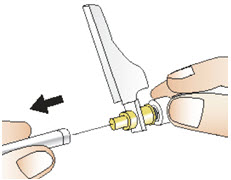
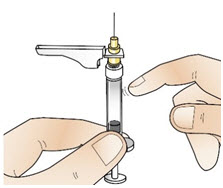
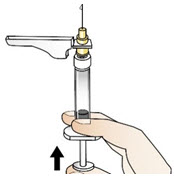
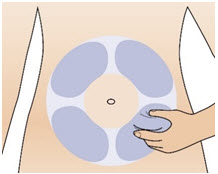
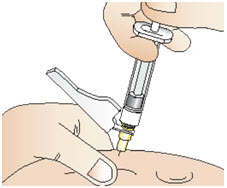
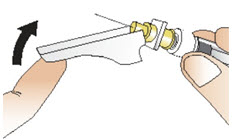
INSTRUCTIONS FOR USEInstructions for Use - CABLIVI® (cab-LIV-ee) (caplacizumab-yhdp) for injection - Single-dose vial - This Instructions for Use has been approved by the U.S. Food and Drug Administration. Be ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 58468-0225-1 - Rx only - Cablivi® caplacizumab-yhdp - for Injection - For Intravenous and Subcutaneous Use - Must be reconstituted with diluent provided. Single-dose vial. Discard unused ...
-
INGREDIENTS AND APPEARANCEProduct Information