Label: CALCITRENE- calcipotriene ointment
- NDC Code(s): 51672-5278-3, 51672-5278-4, 51672-5278-5
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFOR TOPICAL DERMATOLOGIC USE ONLY. Not for Ophthalmic, Oral or Intravaginal Use. Rx Only
-
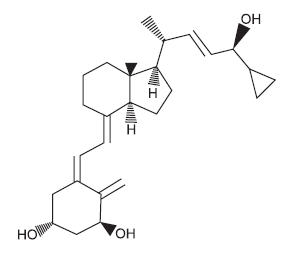
DESCRIPTIONCalcitrene® (calcipotriene) ointment, 0.005% contains the compound calcipotriene, a synthetic vitamin D3 derivative for topical dermatological use. Chemically, calcipotriene is ...
-
CLINICAL PHARMACOLOGYIn humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol) in the skin ...
-
CLINICAL STUDIESAdequate and well-controlled trials of patients treated with Calcitrene® (calcipotriene) ointment, 0.005% have demonstrated improvement usually beginning after two weeks of therapy. This ...
-
INDICATIONS AND USAGECalcitrene® (calcipotriene) ointment, 0.005%, is indicated for the treatment of plaque psoriasis in adults. The safety and effectiveness of topical calcipotriene in dermatoses other than psoriasis ...
-
CONTRAINDICATIONSCalcitrene® (calcipotriene) ointment, 0.005%, is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation. It should not be used by patients ...
-
PRECAUTIONSGeneral - Use of calcipotriene may cause irritation of lesions and surrounding uninvolved skin. If irritation develops, calcipotriene should be discontinued. For external use only. Keep out of ...
-
ADVERSE REACTIONSIn controlled clinical trials, the most frequent adverse reactions reported for calcipotriene were burning, itching and skin irritation, which occurred in approximately 10-15% of patients ...
-
OVERDOSAGETopically applied calcipotriene can be absorbed in sufficient amounts to produce systemic effects. Elevated serum calcium has been observed with excessive use of Calcitrene® (calcipotriene ...
-
DOSAGE AND ADMINISTRATIONApply a thin layer of Calcitrene® (calcipotriene) ointment, 0.005% once or twice daily and rub in gently and completely.
-
HOW SUPPLIEDCalcitrene® (calcipotriene) ointment, 0.005% is available in: 60 gram aluminum tube NDC (51672-5278-3) 120 gram aluminum tube NDC (51672-5278-4) STORAGE - Store at controlled room ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Glenmark Generics Ltd. Colvale-Bardez, Goa 403 513, India - Distributed by: TaroPharma a division of Taro Pharmaceuticals U.S.A., Inc. Hawthorne, NY 10532 - TaroPharma® and ...
-
PRINCIPAL DISPLAY PANEL - 120 g Tube CartonNDC 51672-5278-4 - 120 g - Calcitrene® Calcipotriene Ointment - 0.005% Rx only - TaroPharma® FOR TOPICAL DERMATOLIGIC USE ONLY. NOT FOR OPHTHALMIC, ORAL OR INTRAVAGINAL USE. Keep this and all ...
-
INGREDIENTS AND APPEARANCEProduct Information