Label: CAFCIT- caffeine citrate injection
- NDC Code(s): 0641-6164-01, 0641-6164-10
- Packager: Hikma Pharmaceuticals USA Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
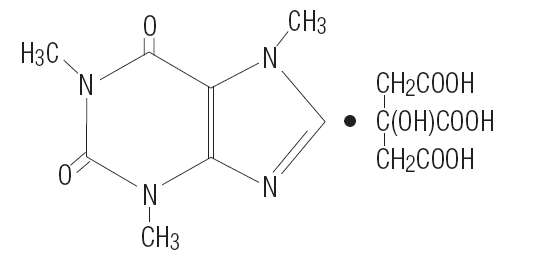
DESCRIPTIONCAFCIT® Injection (caffeine citrate injection, USP) for intravenous administration is a clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solution adjusted to pH 4.7. Each mL ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Caffeine is structurally related to other methylxanthines, theophylline, and theobromine. It is a bronchial smooth muscle relaxant, a CNS stimulant, a cardiac muscle ...
-
INDICATIONS AND USAGECAFCIT (caffeine citrate) is indicated for the treatment of apnea of prematurity.
-
CONTRAINDICATIONSCAFCIT (caffeine citrate) is contraindicated in patients who have demonstrated hypersensitivity to any of its components.
-
WARNINGSNecrotizing Enterocolitis - During the double-blind, placebo-controlled clinical trial, 6 cases of necrotizing enterocolitis developed among the 85 infants studied (caffeine=46, placebo=39), with ...
-
PRECAUTIONSGeneral - Apnea of prematurity is a diagnosis of exclusion. Other causes of apnea (e.g., central nervous system disorders, primary lung disease, anemia, sepsis, metabolic disturbances ...
-
ADVERSE REACTIONSOverall, the reported number of adverse events in the double-blind period of the controlled trial was similar for the CAFCIT (caffeine citrate) and placebo groups. The following table shows ...
-
OVERDOSAGEFollowing overdose, serum caffeine levels have ranged from approximately 24 mg/L (a postmarketing spontaneous case report in which an infant exhibited irritability, poor feeding, and insomnia) to ...
-
DOSAGE AND ADMINISTRATIONPrior to initiation of CAFCIT (caffeine citrate), baseline serum levels of caffeine should be measured in infants previously treated with theophylline, since preterm infants metabolize ...
-
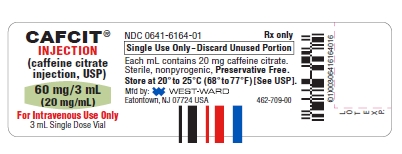
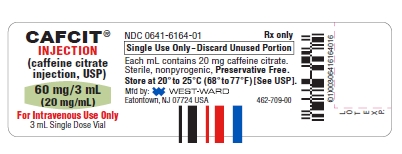
HOW SUPPLIEDCAFCIT® Injection (caffeine citrate injection, USP) is available as a clear, colorless, sterile, non-pyrogenic, preservative-free, aqueous solution in 3 mL colorless glass vials. The vials are ...
-
PRINCIPAL DISPLAY PANELSCAFCIT® INJECTION - (caffeine citrate - injection, USP) 60 mg/3 mL - (20 mg/mL) For Intravenous Use Only - 3 mL Single Dose Vial - NDC 0641-6164-10 Rx only - CAFCIT® INJECTION - (caffeine ...
-
INGREDIENTS AND APPEARANCEProduct Information