Label: BROMSITE 0.075%- bromfenac solution/ drops
- NDC Code(s): 49708-754-41
- Packager: Sun Pharmaceutical Industries, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BROMSITE® safely and effectively. See full prescribing information for BROMSITE. BROMSITE (bromfenac ophthalmic solution) 0.075%, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBromSite (bromfenac ophthalmic solution) 0.075% is indicated for the treatment of postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - One drop of BromSite should be applied to the affected eye twice daily (morning and evening) 1 day prior to surgery, the day of surgery, and 14 days postsurgery. 2.2 ...
-
3 DOSAGE FORM AND STRENGTHSTopical ophthalmic solution: bromfenac 0.075%.
-
4 CONTRAINDICATIONSNone
-
5 WARNINGS AND PRECAUTIONS5.1 Slow or Delayed Healing - All topical nonsteroidal anti-inflammatory drugs (NSAIDs), including BromSite (bromfenac ophthalmic solution) 0.075%, may slow or delay healing. Topical ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described elsewhere in the labeling: • Slow or Delayed Healing [see Warnings and Precautions (5.1)] • Potential for Cross-Sensitivity [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies in pregnant women to inform any drug associated risks. Treatment of pregnant rats and rabbits with oral bromfenac ...
-
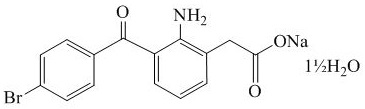
11 DESCRIPTIONBromSite (bromfenac ophthalmic solution) 0.075% is a sterile aqueous, topical NSAID, formulated in DuraSite® for ophthalmic use. The USAN name for bromfenac sodium sesquihydrate is bromfenac ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bromfenac is a nonsteroidal anti-inflammatory drug (NSAID) that has anti-inflammatory activity. The mechanism of its action is thought to be due to its ability to block ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility - Long-term carcinogenicity studies in rats and mice given oral doses of bromfenac up to 0.6 mg/kg/day (129 times a unilateral daily ...
-
14 CLINICAL STUDIES14.1 Ocular Inflammation and Pain - Clinical efficacy was evaluated in 2 multi-centered, randomized, double-masked, parallel group, placebo-controlled US trials in which subjects requiring ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBromSite (bromfenac ophthalmic solution) 0.075% is supplied in white opaque low density polyethylene (LDPE) plastic bottles and translucent dropper tips, and gray high density polyethylene (HDPE ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Slow or Delayed Healing - Advise patients of the possibility that slow or delayed healing may occur while ...
-
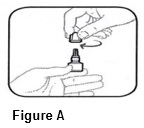
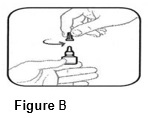
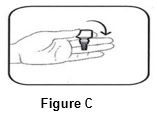
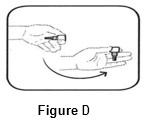
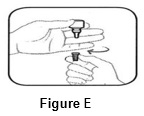
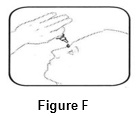
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - BromSite®[BRŏM- sahyt] (bromfenac ophthalmic solution) 0.075% Read this Instructions for Use before you start using BromSite and each time you get a refill. There may be new ...
-
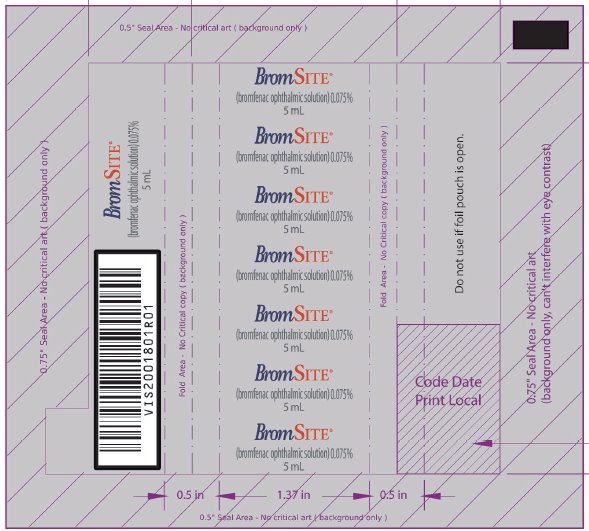
PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML FOIL WRAPPER
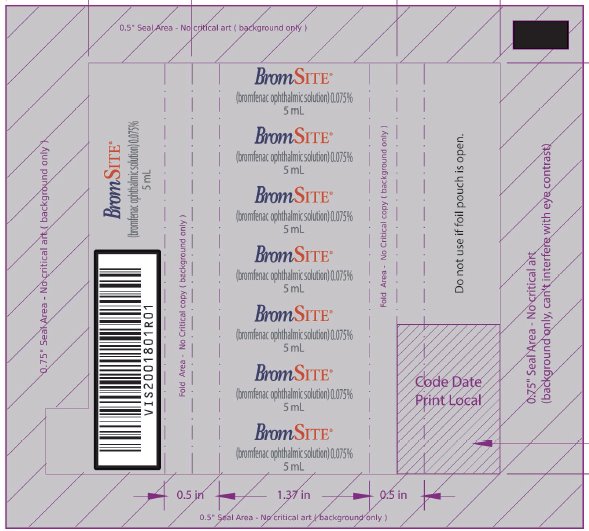
-
PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML BOTTLE LABEL

-
PRINCIPAL DISPLAY PANEL - NDC: 49708-754-41 - 5 ML CARTON LABEL

-
INGREDIENTS AND APPEARANCEProduct Information