Label: BEOVU- brolucizumab injection, solution
- NDC Code(s): 0078-0827-60, 0078-0827-61, 0078-0827-98, 0078-0827-99
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated February 24, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BEOVU safely and effectively. See full prescribing information for BEOVU. BEOVU® (brolucizumab-dbll) injection, for intravitreal ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBEOVU® is indicated for the treatment of: 1.1 Neovascular (Wet) Age-related Macular Degeneration (AMD) 1.2 Diabetic Macular Edema (DME)
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - For ophthalmic intravitreal injection. BEOVU must be administered by a qualified physician. BEOVU is available packaged as follows [see How Supplied/Storage ...
-
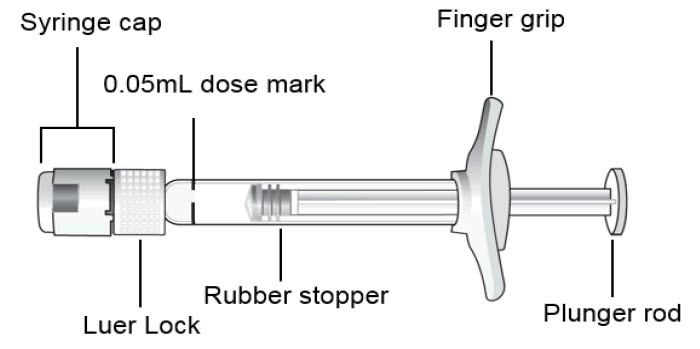
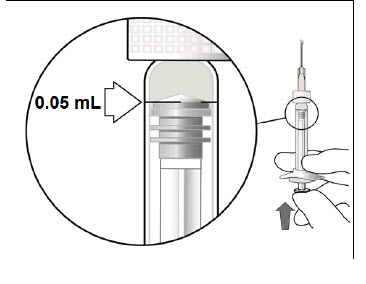
3 DOSAGE FORMS AND STRENGTHSBEOVU is a clear to slightly opalescent and colorless to slightly brownish-yellow solution available as: Intravitreal injection: 6 mg/0.05 mL in a single-dose pre-filled syringe. Intravitreal ...
-
4 CONTRAINDICATIONS4.1 Ocular or Periocular Infections - BEOVU is contraindicated in patients with ocular or periocular infections. 4.2 Active Intraocular Inflammation - BEOVU is contraindicated in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Endophthalmitis and Retinal Detachment - Intravitreal injections, including those with BEOVU, have been associated with endophthalmitis and retinal detachment [see Contraindications (4.1 ...
-
6 ADVERSE REACTIONSThe following potentially serious adverse reactions are described elsewhere in the labeling: Hypersensitivity [see Contraindications (4.3)] Endophthalmitis and Retinal Detachment [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies of BEOVU administration in pregnant women. In an animal reproduction study, intravitreal administration of ...
-
11 DESCRIPTIONBrolucizumab-dbll is a recombinant human vascular endothelial growth factor inhibitor. Brolucizumab-dbll is a humanized monoclonal single-chain Fv (scFv) antibody fragment. Brolucizumab-dbll has a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Brolucizumab is a human VEGF inhibitor. Brolucizumab binds to the three major isoforms of VEGF-A (e.g., VEGF110, VEGF121, and VEGF165), thereby preventing ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies have been conducted on the carcinogenic or mutagenic potential of BEOVU. Based on the anti-VEGF mechanism of action ...
-
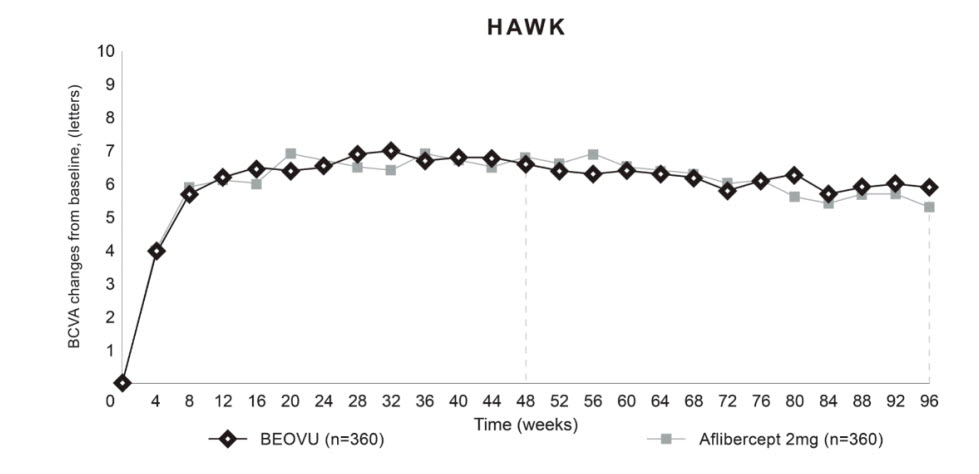
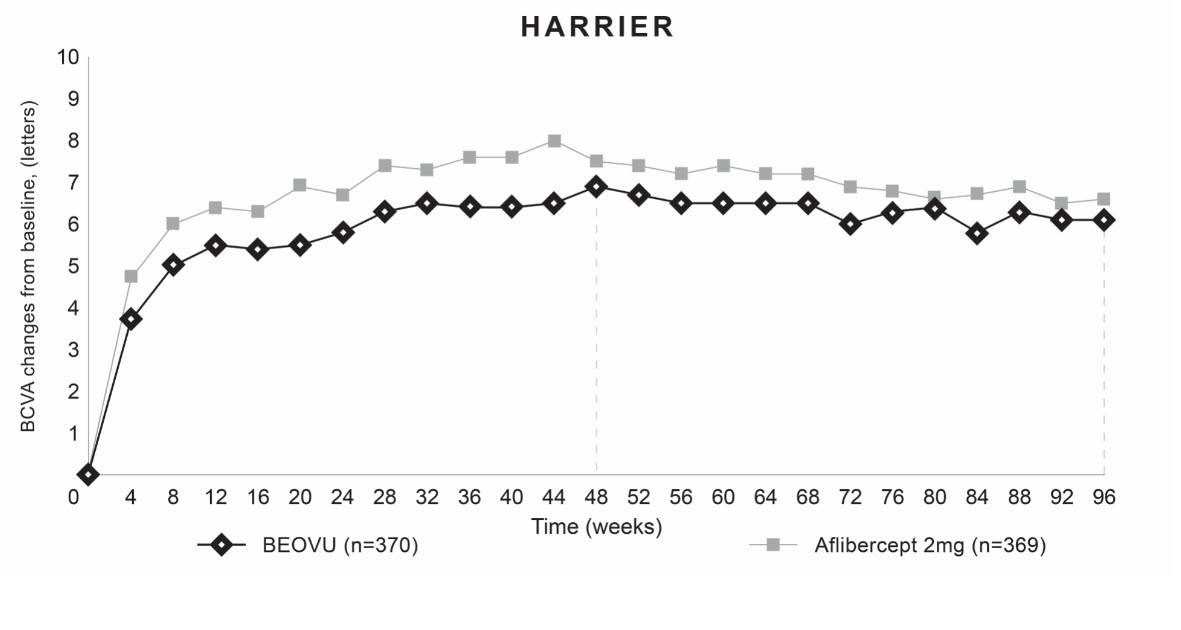
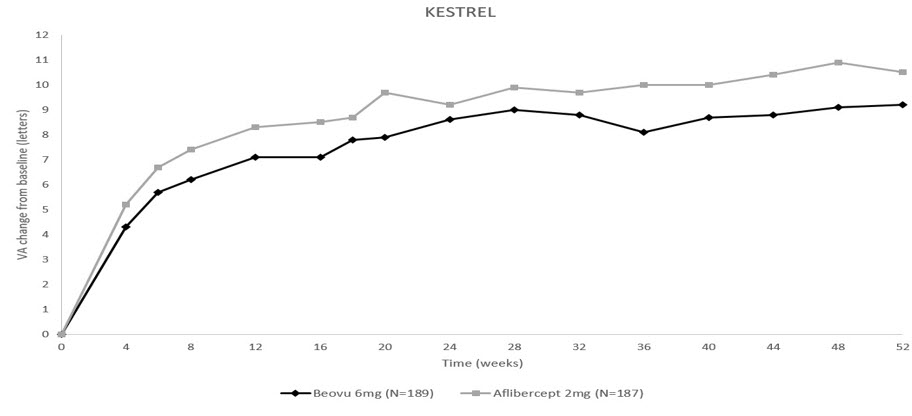
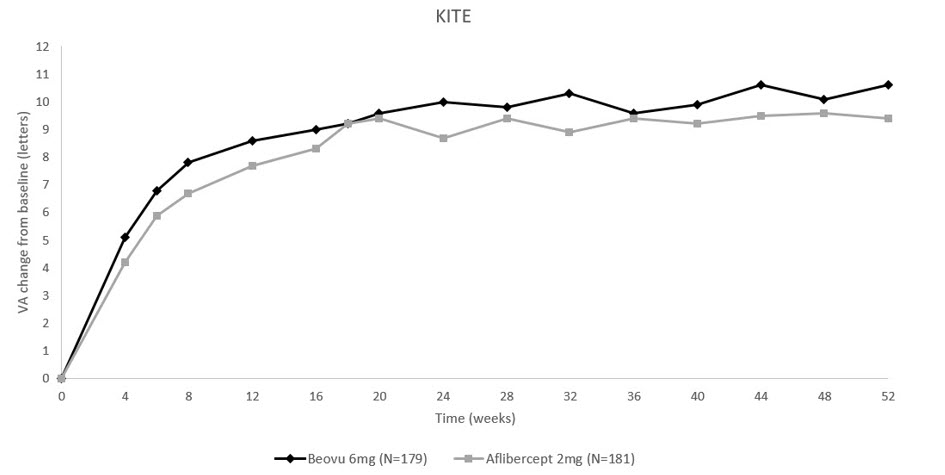
14 CLINICAL STUDIES14.1 Neovascular (Wet) Age-Related Macular Degeneration (AMD) The safety and efficacy of BEOVU were assessed in two randomized, multi-center, double-masked, active-controlled studies (HAWK ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - BEOVU (brolucizumab-dbll) injection is supplied as a clear to slightly opalescent and colorless to slightly brownish-yellow solution in a single-dose pre-filled syringe ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients that in the days following BEOVU administration, patients are at risk of developing endophthalmitis, retinal detachment, retinal vasculitis and/or retinal vascular occlusion. If ...
-
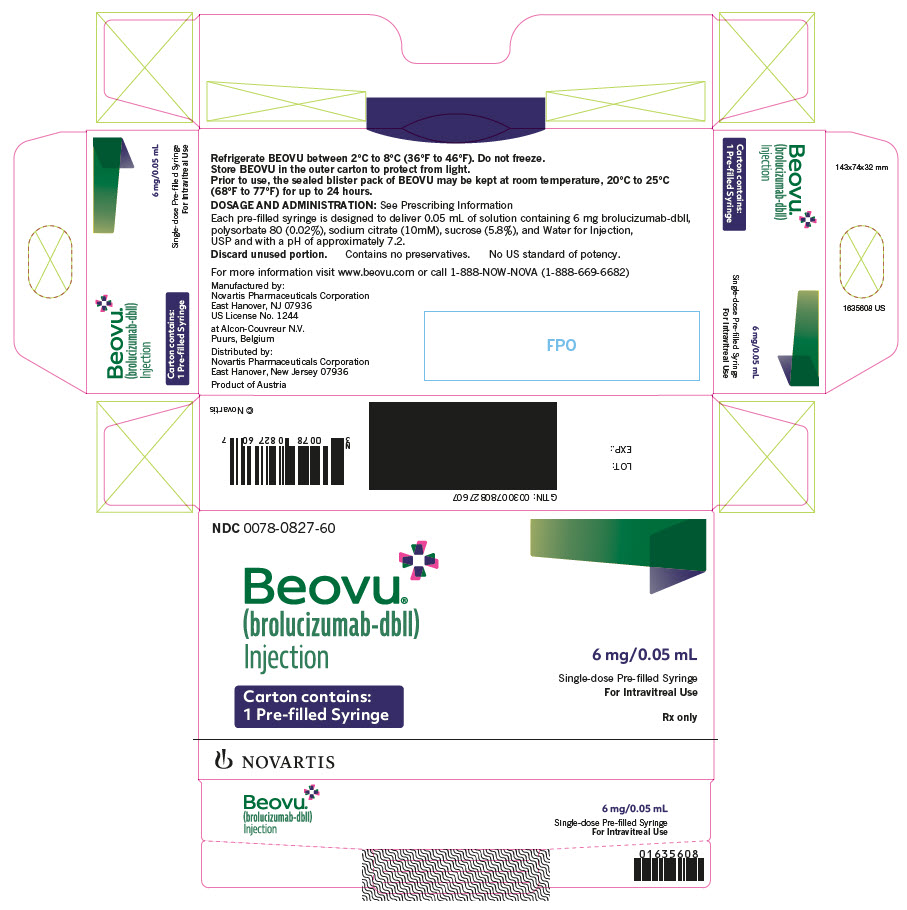
PRINCIPAL DISPLAY PANELNDC 0078-0827-60 - Beovu® (brolucizumab-dbll) Injection - 6 mg/0.05 mL - Single-dose Pre-filled Syringe - For Intravitreal Use - Carton contains: 1 Pre-filled Syringe - Rx only - NOVARTIS
-
INGREDIENTS AND APPEARANCEProduct Information

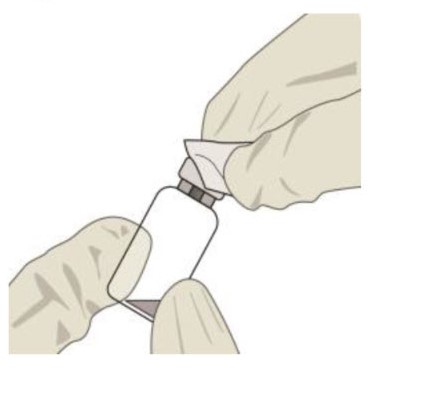
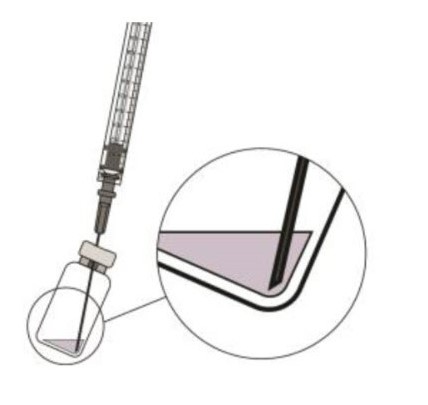
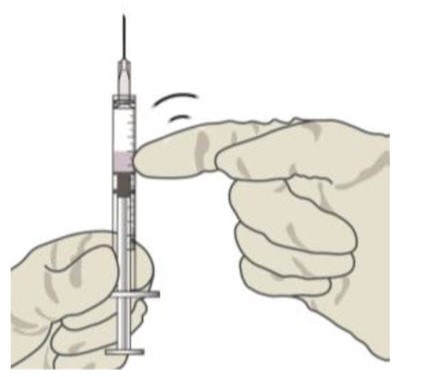
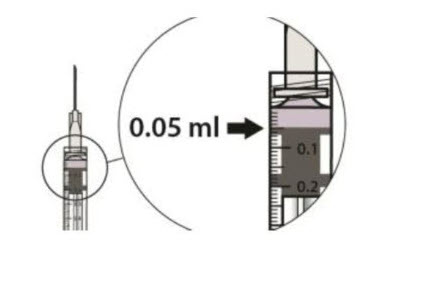
![Preparation for Administration - BEOVU should be inspected visually upon removal from the refrigerator and prior to administration. If particulates, cloudiness, or discoloration are visible, the glass vial must not be used. The BEOVU kit includes the sterile glass vial and filter needle which are for single use only. Do not use if the packaging, vial and/or filter needle are damaged or expired [see How Supplied/Storage and Handling (16)].](/dailymed/image.cfm?name=rth258-04.jpg&setid=5d1dc1fa-a2d3-46ed-9e9a-c1a036590d3d)