Label: BIDIL- hydralazine hydrochloride and isosorbide dinitrate tablet, film coated
- NDC Code(s): 24338-010-09, 24338-010-12, 24338-010-18
- Packager: Azurity
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 19, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BIDIL safely and effectively. See full prescribing information for BIDIL. BIDIL® (isosorbide dinitrate and hydralazine ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Treatment of Heart Failure in Self-identified Black Patients - BiDil is indicated for the treatment of heart failure as an adjunct to standard therapy in self-identified black patients to ...
-
2 DOSAGE AND ADMINISTRATIONBiDil should be initiated at a dose of one BiDil Tablet, three times a day. Titrate to a maximum of two tablets three times daily, if tolerated. Although titration of BiDil can be rapid (3-5 ...
-
3 DOSAGE FORMS AND STRENGTHSThe BiDil (20 mg isosorbide dinitrate and 37.5 mg hydralazine hydrochloride) tablets are orange, biconvex, approximately 8 mm in diameter, scored, film-coated, and debossed with "20" on one side ...
-
4 CONTRAINDICATIONSBiDil is contraindicated in patients who are allergic to organic nitrates. Do not use BiDil in patients who are taking PDE-5 inhibitors, such as avanafil, sildenafil, tadalafil, or vardenafil ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Symptomatic hypotension, particularly with upright posture, may occur with even small doses of BiDil. Hypotension is most likely to occur in patients who have been volume or ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Phosphodiesterase Inhibitors - BiDil is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), PDE5 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on BiDil use in pregnant women, and insufficient data on its components (hydralazine and isosorbide dinitrate) to assess a drug-associated risk ...
-
10 OVERDOSAGEThe signs and symptoms of overdosage with BiDil are expected to be those of excessive pharmacologic effect, i.e., vasodilatation, reduced cardiac output and hypotension, and signs and symptoms ...
-
11 DESCRIPTIONBiDil is a fixed-dose combination of isosorbide dinitrate, a vasodilator with effects on both arteries and veins, and hydralazine hydrochloride, a predominantly arterial vasodilator. Isosorbide ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action underlying the beneficial effects of BiDil in the treatment of heart failure has not been established. Isosorbide dinitrate is a vasodilator ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Hydralazine hydrochloride: An increased incidence of lung tumors (adenomas and adenocarcinomas) was observed in a lifetime study ...
-
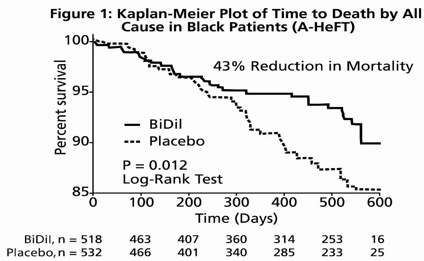
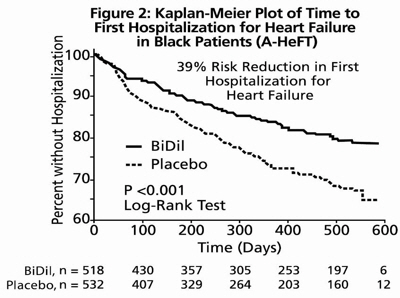
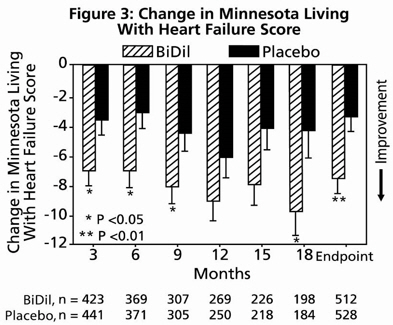
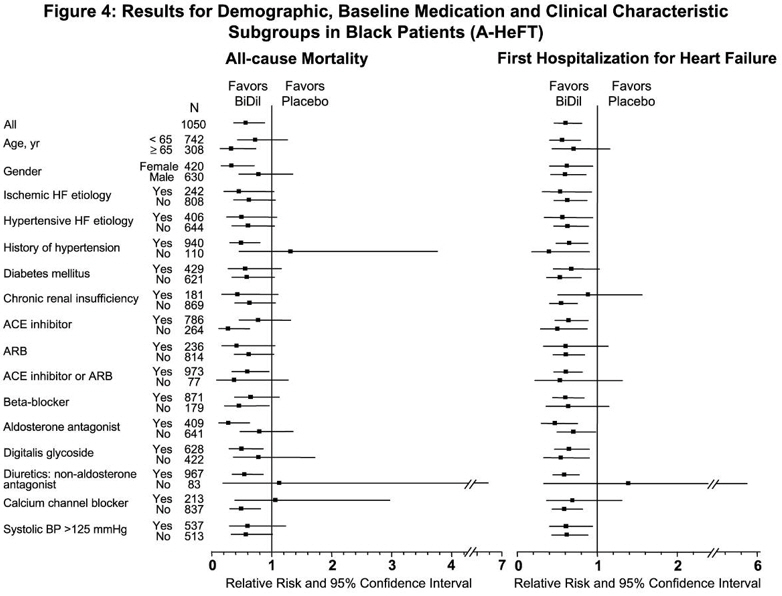
14 CLINICAL STUDIESBiDil or a combination of isosorbide dinitrate and hydralazine hydrochloride was studied in two placebo-controlled clinical trials in 1,692 patients with mild to severe heart failure (mostly NYHA ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBiDil Tablets contain 20 mg of isosorbide dinitrate and 37.5 mg of hydralazine hydrochloride. They are biconvex, approximately 8 mm in diameter, scored, film-coated, orange tablets debossed "20 ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be informed of possible side effects and advised to take the medication regularly and continuously as directed. Headache - Inform patients that headaches often accompany ...
-
SPL UNCLASSIFIED SECTIONManufactured for: arbor® PHARMACEUTICALS, LLC - Atlanta, GA 30328 - Manufactured by: Lannett Company, Inc. Philadelphia, PA 19136 - BiDil is a registered trademark of Arbor Pharmaceuticals, LLC - ...
-
PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle LabelNDC 24338-010-09 - 90 tablets - BiDil® isosorbide dinitrate and hydralazine HCl tablets - 20 mg/37.5 mg - Store at room temperature 15°C - 30°C (59°F - 86°F) BDL-TL-09-00 Rev. 09/20 - CIB71996B - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information